WO1999045954A1 - Hla-binding peptides and their uses - Google Patents
Hla-binding peptides and their uses Download PDFInfo
- Publication number
- WO1999045954A1 WO1999045954A1 PCT/US1998/005039 US9805039W WO9945954A1 WO 1999045954 A1 WO1999045954 A1 WO 1999045954A1 US 9805039 W US9805039 W US 9805039W WO 9945954 A1 WO9945954 A1 WO 9945954A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- hbv
- peptide
- pol
- hiv
- sequence
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/195—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from bacteria
- C07K14/36—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from bacteria from Actinomyces; from Streptomyces (G)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/10—Immunoglobulins specific features characterized by their source of isolation or production
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
Definitions
- the present invention relates to compositions and methods for preventing, treating or diagnosing a number of pathological states such as viral diseases and cancers.
- it provides novel peptides capable of binding selected major histocompatibility complex (MHC) molecules and inducing an immune response.
- MHC major histocompatibility complex
- Class I MHC molecules are classified as either Class I or Class II molecules.
- Class II MHC molecules are expressed primarily on cells involved in initiating and sustaining immune responses, such as T lymphocytes, B lymphocytes, macrophages, etc.
- Class II MHC molecules are recognized by helper T lymphocytes and induce proliferation of helper T lymphocytes and amplification of the immune response to the particular immunogenic peptide that is displayed.
- Class I MHC molecules are expressed on almost all nucleated cells and are recognized by cytotoxic T lymphocytes (CTLs), which then destroy the antigen-bearing cells. CTLs are particularly important in tumor rejection and in fighting viral infections.
- CTLs cytotoxic T lymphocytes
- the CTL recognizes the antigen in the form of a peptide fragment bound to the MHC class I molecules rather than the intact foreign antigen itself.
- the antigen must normally be endogenously synthesized by the cell, and a portion of the protein antigen is degraded into small peptide fragments in the cytoplasm. Some of these small peptides translocate into a pre-Golgi compartment and interact with class I heavy chains to facilitate proper folding and association with the subunit ⁇ 2 microglobulin.
- the peptide-MHC class I complex is then routed to the cell surface for expression and potential recognition by specific CTLs.
- compositions comprising immunogenic peptides having binding motifs for HLA molecules.
- the immunogenic peptides which bind to the appropriate MHC allele, comprise conserved residues at certain positions which allow the peptides to bind desired HLA molecules.
- PSA prostate cancer specific antigen
- HBVc hepatitis B core and surface antigens
- HBVs hepatitis C antigens
- Epstein-Barr virus antigens Epstein-Barr virus antigens
- HMV1 human immunodeficiency type-1 virus
- Kaposi's sarcoma herpes virus KSHV
- human papilloma virus HPV
- HPV human papilloma virus
- Lassa virus Lassa virus
- mycobacterium tuberculosis MT
- CEA trypanosome surface antigen
- Her2/neu Her2/neu.
- the peptides are thus useful in pharmaceutical compositions for both therapeutic and diagnostic applications.
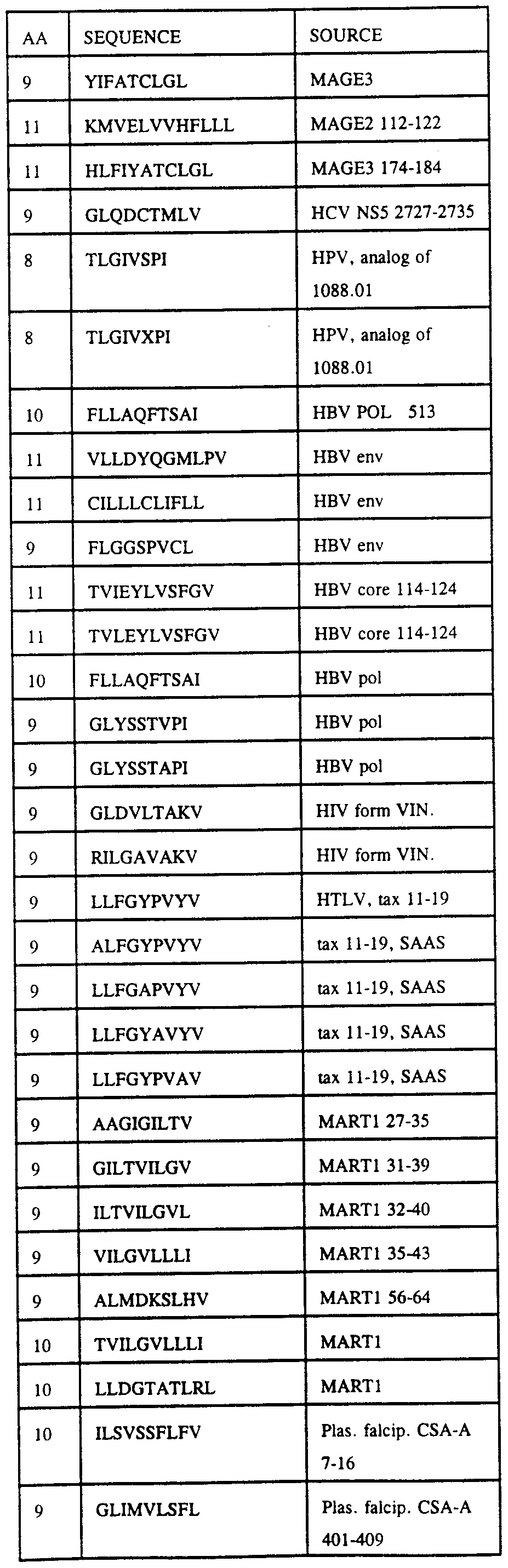
- the invention provides compositions comprising an immunogenic peptide having an HLA binding motif, which immunogenic peptide is a peptide shown in Tables 3-14. Also provided are peptides comprising a conservative substitution of a residue in a peptide shown in Table 3-14.
- the immunogenic peptide of the invention can be further linked to a second oligopeptide. In some embodiments, the second oligopeptide is a peptide that induces a helper T response.
- the invention further provides nucleic acid molecules encoding immunogenic peptides as shown in Tables 3-14, or peptides comprising a conservative substitution of a residue of a peptide shown in Table 3-14.
- the nucleic acid may further comprise a sequence encoding a second immunogenic peptide or peptide that induces a helper T response.
- the peptides provided here can be used to induce a cytotoxic T cell response either in vivo or in vitro.
- the methods comprise contacting a cytotoxic T cell with a peptide of the invention.
- peptide is used interchangeably with “oligopeptide” in the present specification to designate a series of residues, typically L- amino acids, connected one to the other typically by peptide bonds between the alpha-amino and carbonyl groups of adjacent amino acids.
- the oligopeptides of the invention are less than about 15 residues in length and usually consist of between about 8 and about 11 residues, preferably 9 or 10 residues.
- An "immunogenic peptide” is a peptide which comprises an allele-specific motif such that the peptide will bind an MHC molecule and induce a CTL response.
- Immunogenic peptides of the invention are capable of binding to an appropriate HLA molecule and inducing a cytotoxic T cell response against the antigen from which the immunogenic peptide is derived.
- Immunogenic peptides are conveniently identified using the algorithms of the invention.
- the algorithms are mathematical procedures that produce a score which 4 enables the selection of immunogenic peptides.
- the algorithm is based upon either the effects on MHC binding of a particular amino acid at a particular position of a peptide or the effects on binding of a particular substitution in a motif containing peptide.
- a “conserved residue” is an amino acid which occurs in a significantly higher frequency than would be expected by random distribution at a particular position in a peptide.
- a conserved residue is one where the MHC structure may provide a contact point with the immunogenic peptide.
- At least one to three or more, preferably two, conserved residues within a peptide of defined length defines a motif for an immunogenic peptide. These residues are typically in close contact with the peptide binding groove, with their side chains buried in specific pockets of the groove itself.
- an immunogenic peptide will comprise up to three conserved residues, more usually two conserved residues.
- "negative binding residues" are amino acids which if present at certain positions will result in a peptide being a nonbinder or poor binder and in turn fail to be immunogenic i.e. induce a CTL response.
- motif refers to the pattern of residues in a peptide of defined length, usually about 8 to about 11 amino acids, which is recognized by a particular MHC allele.
- the peptide motifs are typically different for each human MHC allele and differ in the pattern of the highly conserved residues and negative residues.
- the binding motif for an allele can be defined with increasing degrees of precision. In one case, all of the conserved residues are present in the correct positions in a peptide and there are no negative residues in positions 1,3 and/or 7.
- the phrases "isolated” or “biologically pure” refer to material which is substantially or essentially free from components which normally accompany it as found in its native state. Thus, the peptides of this invention do not contain materials normally associated with their in situ environment, e.g. , MHC I molecules on antigen presenting cells. Even where a protein has been isolated to a homogenous or dominant band, there are trace contaminants in the range of 5-10% of native protein which co-purify with the desired protein. Isolated peptides of this invention do not contain such endogenous co- purified protein. 5
- residue refers to an amino acid or amino acid mimetic incorporated in an oligopeptide by an amide bond or amide bond mimetic.
- the present invention relates to the determination of allele-specific peptide motifs for human Class I MHC (sometimes referred to as HLA) allele subtypes, in particular, peptide motifs recognized by HLA alleles.
- HLA human Class I MHC
- a peptide of 9 amino acids preferrably has the following motif: a first conserved residue at the second position from the N-terminus selected from the group consisting of I, V, A and T and a second conserved residue at the C-terminal position selected from the group consisting of V, L, I, A and M.
- An alternate motif is one in which the first conserved residue at the second position from the N- terminus selected is from the group consisting of L, M, I, V, A and T and the second conserved residue at the C-terminal position selected from the group consisting of A and M .
- the amino acid at position 1 is preferrably not an amino acid selected from the group consisting of D, and P.
- the amino acid at position 3 from the N-terminus is not an amino acid selected from the group consisting of D, E, R, K and H.
- the amino acid at position 6 from the N-terminus is not an amino acid selected from the group consisting of R, K and H .
- the amino acid at at position 7 from the N-terminus is not an amino acid selected from the group consisting of R, K, H, D and E.
- the HLA-A2.1 binding motif for peptide of 10 residues is as follows: a first conserved residue at the second position from the N-terminus selected from the group consisting of L, M, I, V, A, and T, and a second conserved residue at the C-terminal position selected from the group consisting of V, I, L, A and M.
- the first and second conserved residues are separated by 7 residues.
- the amino acid at position 1 is not an amino acid selected from the group consisting of D, E and P.
- the N-terminal residue is not an amino acid selected from the group consisting of D and E.
- the residue at position 4 from the N-terminus is not an amino acid selected from the group consisting of A, K, R and H.
- the amino acid at positon 5 from the N-terminus is not P.
- the amino acid at position 7 from the N-terminus is not an amino acid selected from the group consisting of R, K .and H.
- the amino acid at position 8 from the N-terminus is not amino acid selected from the group consisting of D, E, R, K and H.
- 9 from the N-terminus is not an amino acid selected from the group consisting of R, K and
- Te motif for HLA-A3.2 comprises from the N-terminus to C-terminus a first conserved residue of L, M, I, V, S, A, T and F at position 2 and a second conserved residue of K, R or Y at the C-terminal end.
- Other first conserved residues are C, G or D and alternatively E.
- Other second conserved residues are H or F.
- the first and second conserved residues are preferably separated by 6 to 7 residues.
- the motif for HLA-A1 comprises from the N-terminus to the C-terminus a first conserved residue of T, S or M, a second conserved residue of D or E, and a third conserved residue of Y.
- Other second conserved residues are A, S or T.
- the first and second conserved residues are adjacent and are preferably separated from the third conserved residue by 6 to 7 residues.
- a second motif consists of a first conserved residue of E or D and a second conserved residue of Y where the first and second conserved residues are separated by 5 to 6 residues.
- the motif for HLA-A11 comprises from the N-terminus to the C-terminus a first conserved residue of T, V, M, L, I, S, A, G, N, C D, or F at position 2 and a C- terminal conserved residue of K, R, Y or H.
- the first and second conserved residues are preferably separated by 6 or 7 residues.
- the motif for HLA-A24.1 comprises from the N-terminus to the C-terminus a first conserved residue of Y, F or W at position 2 and a C terminal conserved residue of
- the first and second conserved residues are preferably separated by 6 to
- T cell epitopes from any desired antigen, particularly those associated with human viral diseases, cancers or autoiummune diseases, for which the amino acid sequence of the potential antigen or autoantigen targets is known.
- PSA prostate specific antigen
- HBVc hepatitis B core and surface antigens
- HBVs hepatitis C antigens
- Epstein-Barr virus antigens Epstein-Barr virus antigens
- melanoma antigens e.g., MAGE-1
- HAV human immunodeficiency virus
- HPV human papilloma virus
- Lassa virus mycobacterium tuberculosis
- CEA trypanosome surface antigen
- Her2/neu Her2/neu.
- Peptides comprising the epitopes from these antigens are synthesized and then tested for their ability to bind to the appropriate MHC molecules in assays using, for example, purified class I molecules and radioiodonated peptides and/or cells expressing empty class I molecules by, for instance, immunofluorescent staining and flow microfluorometry, peptide-dependent class I assembly assays, and inhibition of CTL recognition by peptide competition.
- Those peptides that bind to the class I molecule are further evaluated for their ability to serve as targets for CTLs derived from infected or immunized individuals, as well as for their capacity to induce primary in vitro or in vivo CTL responses that can give rise to CTL populations capable of reacting with virally infected target cells or tumor cells as potential therapeutic agents.
- the MHC class I antigens are encoded by the HLA-A, B, and C loci.
- HLA-A and B antigens are expressed at the cell surface at approximately equal densities, whereas the expression of HLA-C is significantly lower (perhaps as much as 10-fold lower).
- Each of these loci have a number of alleles.
- the peptide binding motifs of the invention are relatively specific for each allelic subtype.
- the peptides of the present invention preferably comprise a motif recognized by an MHC I molecule having a wide distribution in the human population. Since the MHC alleles occur at different frequencies within different ethnic groups and races, the choice of target MHC allele may depend upon the target population. Table 1 shows the frequency of various alleles at the HLA-A locus products among different races. For instance, the majority of the Caucasoid population can be covered by peptides which bind to four HLA-A allele subtypes, specifically HLA-A2.1 , Al, A3.2, and A24.1. Similarly, the majority of the Asian population is encompassed with the addition of peptides binding to a fifth allele HLA- A 11.2.
- N negroid
- A Asian
- C caucasoid. Numbers in parenthesis represent the number of individuals included in the analysis.
- each residue is generally represented by standard three letter or single letter designations.
- the L-form of an amino acid residue is represented by a capital single letter or a capital first letter of a three-letter symbol, and the D-form for those amino acids having D-forms is represented by a lower case single letter or a lower case three letter symbol.
- Gly cine has no asymmetric carbon atom and is simply referred to as "Gly" or G.
- the procedures used to identify peptides of the present invention generally follow the methods disclosed in Falk et al. , Nature 351:290 (1991), which is incorporated herein by reference. Briefly, the methods involve large-scale isolation of MHC class I molecules, typically by immunoprecipitation or affinity chromatography, from the appropriate cell or cell line. Examples of other methods for isolation of the desired MHC molecule equally well known to the artisan include ion exchange chromatography, lectin chromatography, size exclusion, high performance ligand chromatography, and a combination of all of the above techniques.
- immunoprecipitation is used to isolate the desired allele.
- a number of protocols can be used, depending upon the specificity of the antibodies used.
- allele-specific mAb reagents can be used for the affinity purification of the
- HLA-A, HLA-B,, and HLA-C molecules HLA-A, HLA-B, and HLA-C molecules.
- Several mAb reagents for the isolation of HLA-A molecules are available.
- the monoclonal BB7.2 is suitable for isolating HLA-A2 molecules.
- Affinity columns prepared with these mAbs using standard techniques are successfully used to purify the respective HLA-A allele products.
- broadly reactive anti-HLA-A, B, C mAbs, such as W6/32 and B9.12.1, and one anti-HLA-B, C mAb, B 1.23.2 could be used in alternative affinity purification protocols as described in previous applications.
- the peptides bound to the peptide binding groove of the isolated MHC molecules are eluted typically using acid treatment.
- Peptides can also be dissociated from class I molecules by a variety of standard denaturing means, such as heat, pH, detergents, salts, chaotropic agents, or a combination thereof. 10
- Peptide fractions are further separated from the MHC molecules by reversed-phase high performance liquid chromatography (HPLC) and sequenced.
- HPLC high performance liquid chromatography
- Peptides can be separated by a variety of other standard means well known to the artisan, including filtration, ultrafiltration, electrophoresis, size chromatography, precipitation with specific antibodies, ion exchange chromatography, isoelectrofocusing, and the like.
- Sequencing of the isolated peptides can be performed according to standard techniques such as Edman degradation (Hunkapiller, M.W. , et al.. Methods Enzymol. 91, 399 [1983]). Other methods suitable for sequencing include mass spectrometry sequencing of individual peptides as previously described (Hunt, et al., Science 225: 1261 (1992), which is incorporated herein by reference). Amino acid sequencing of bulk heterogenous peptides (e.g.. pooled HPLC fractions) from different class I molecules typically reveals a characteristic sequence motif for each class I allele.
- motifs specific for different class I alleles allows the identification of potential peptide epitopes from an antigenic protein whose amino acid sequence is known. Typically, identification of potential peptide epitopes is initially carried out using a computer to scan the amino acid sequence of a desired antigen for the presence of motifs. The epitopic sequences are then synthesized. The capacity to bind MHC Class molecules is measured in a variety of different ways. One means is a Class I molecule binding assay as described in the related applications, noted above. Other alternatives described in the literature include inhibition of antigen presentation (Sette, et al. , J. Immunol.
- peptides that test positive in the MHC class I binding assay are assayed for the ability of the peptides to induce specific CTL responses in vitro.
- Antigen-presenting cells that have been incubated with a peptide can be assayed for the ability to induce CTL responses in responder cell populations.
- Antigen-presenting cells can be normal cells such as peripheral blood mononuclear cells or dendritic cells (Inaba, et al. , J. Exp. Med. 166: 182 (1987); Boog, Eur. J. Immunol. 18:219 [1988]).
- mutant mammalian cell lines that are deficient in their ability to load class I molecules with internally processed peptides, such as the mouse cell lines RMA-S (Karre, et al.. Nature. 319:675 (1986); Ljunggren, et al., Fur. J. Immunol. 11
- T-2 human somatic T cell hybrid
- Other eukaryotic cell lines which could be used include various insect cell lines such as mosquito larvae (ATCC cell lines
- Peripheral blood lymphocytes are conveniently isolated following simple venipuncture or leukapheresis of normal donors or patients and used as the responder cell sources of CTL precursors.
- the appropriate antigen-presenting cells are incubated with 10-100 ⁇ M of peptide in serum-free media for 4 hours under appropriate culture conditions.
- the peptide-loaded antigen-presenting cells are then incubated with the responder cell populations in vitro for 7 to 10 days under optimized culture conditions.
- Positive CTL activation can be determined by assaying the cultures for the presence of CTLs that kill radiolabeled target cells, both specific peptide-pulsed targets as well as target cells expressing endogenously processed form of the relevant virus or tumor antigen from which the peptide sequence was derived.
- Specificity and MHC restriction of the CTL is determined by testing against different peptide target cells expressing appropriate or inappropriate human MHC class I.
- immunogenic peptides that test positive in the MHC binding assays and give rise to specific CTL responses are referred to herein as immunogenic peptides.
- the immunogenic peptides can be prepared synthetically, or by recombinant DNA technology or from natural sources such as whole viruses or tumors. Although the peptide will preferably be substantially free of other naturally occurring host cell proteins and fragments thereof, in some embodiments the peptides can be synthetically conjugated to native fragments or particles.
- polypeptides or peptides can be a variety of lengths, either in their neutral (uncharged) forms or in forms which are salts, and either free of modifications such as glycosylation, side chain oxidation, or phosphorylation or containing these modifications, subject to the condition that the modification not destroy the biological activity of the polypeptides as herein described. 12
- the peptide will be as small as possible while still maintaining substantially all of the biological activity of the large peptide.
- Peptides having the desired activity may be modified as necessary to provide certain desired attributes, e.g. , improved pharmacological characteristics, while increasing or at least retaining substantially all of the biological activity of the unmodified peptide to bind the desired MHC molecule and activate the appropriate T cell.
- the peptides may be subject to various changes, such as substitutions, either conservative or non-conservative, where such changes might provide for certain advantages in their use, such as improved MHC binding.
- conservative substitutions is meant replacing an amino acid residue with another which is biologically and/or chemically similar, e.g., one hydrophobic residue for another, or one polar residue for another.
- the substitutions include combinations such as Gly, Ala; Val, He, Leu, Met;
- the peptides can also be modified by extending or decreasing the compound's amino acid sequence, e.g. , by the addition or deletion of amino acids.
- the peptides or analogs of the invention can also be modified by altering the order or composition of certain residues, it being readily appreciated that certain amino acid residues essential for biological activity, e.g., those at critical contact sites or conserved residues, may generally not be altered without an adverse effect on biological activity.
- the non-critical amino acids need not be limited to those naturally occurring in proteins, such as L- ⁇ -amino acids, or their D-isomers, but may include non-natural amino acids as well, such as ⁇ - ⁇ - ⁇ -amino acids, as well as many derivatives of L- ⁇ -amino acids.
- a series of positively charged (e.g. , Lys or Arg) or negatively charged (e.g. , Glu) amino acid substitutions are made along the length of the peptide revealing different patterns of sensitivity towards various MHC molecules and T cell receptors.
- multiple substitutions using small, relatively neutral moieties such as Ala, Gly, Pro, or similar residues may be employed.
- the substitutions may be homo-oligomers or hetero- oligomers.
- the number and types of residues which are substituted or added depend on the spacing necessary between essential contact points and certain functional attributes which are sought (e.g., hydrophobicity versus hydrophilicity).
- Increased binding affinity for an MHC molecule or T cell receptor may also be achieved by such substitutions, compared to the affinity of the parent peptide.
- substitutions should employ amino acid residues or other molecular fragments chosen to avoid, for example, steric and charge interference which might disrupt binding.
- Amino acid substitutions are typically of single residues. Substitutions, deletions, insertions or any combination thereof may be combined to arrive at a final peptide. Substitutional variants are those in which at least one residue of a peptide has been removed and a different residue inserted in its place. Such substitutions generally are made in accordance with the following Table 2 when it is desired to finely modulate the characteristics of the peptide.
- Substantial changes in function are made by selecting substitutions that are less conservative than those in Table 2, i.e. , selecting residues that differ more significantly in their effect on maintaining (a) the structure of the peptide backbone in the area of the substitution, for example as a sheet or helical conformation, (b) the charge or hydrophobicity of the molecule at the target site or (c) the bulk of the side chain.
- the substimtions which in general are expected to produce the greatest changes in peptide properties will be those in which (a) hydrophilic residue, e.g. seryl, is substituted for (or by) a hydrophobic residue, e.g.
- leucyl isoleucyl, phenylalanyl, valyl or alanyl
- a residue having an electropositive side chain e.g., lysl, arginyl, or histidyl
- an electronegative residue e.g. glutamyl or aspartyl
- a residue having a bulky side chain e.g. phenylalanine, is substituted for (or by) one not having a side chain, e.g., glycine.
- the peptides may also comprise isosteres of two or more residues in the immunogenic peptide.
- An isostere as defined here is a sequence of two or more residues that can be substituted for a second sequence because the steric conformation of the first sequence fits a binding site specific for the second sequence.
- the term specifically includes peptide backbone modifications well known to those skilled in the art. Such modifications include modifications of the amide nitrogen, the ⁇ -carbon, amide carbonyl, complete replacement of the amide bond, extensions, deletions or backbone crosslinks. See, generally. Spatola, Chemistry and Biochemistry of Amino Acids, peptides and Proteins. Vol. VII (Weinstein ed. , 1983).
- Modifications of peptides with various amino acid mimetics or unnatural amino acids are particularly useful in increasing the stability of the peptide in vivo. Stability can be assayed in a number of ways. For instance, peptidases and various biological media, such as human plasma and serum, have been used to test stability. See, e.g.. Verhoef et al., Eur. J. Drug Metab. Pharmacokin. 11:291-302 (1986). Half life of the peptides of the present invention is conveniently determined using a 25 % human serum (v/v) assay. The protocol is generally as follows. Pooled human serum (Type AB, non-heat inactivated) is delipidated by centrifugation before use.
- Type AB non-heat inactivated
- the serum is then diluted to 25% with RPMI tissue culture media and used to test peptide stability. At predetermined time intervals a small amount of reaction solution is removed and added to either 6% aqueous trichloracetic acid or ethanol. The cloudy reaction sample is cooled 16
- the peptides of the present invention or analogs thereof which have CTL stimulating activity may be modified to provide desired attributes other than improved serum half life.
- the ability of the peptides to induce CTL activity can be enhanced by linkage to a sequence which contains at least one epitope that is capable of inducing a T helper cell response.
- Particularly preferred immunogenic peptides/T helper conjugates are linked by a spacer molecule.
- the spacer is typically comprised of relatively small, neutral molecules, such as amino acids or amino acid mimetics, which are substantially uncharged under physiological conditions.
- the spacers are typically selected from, e.g. , Ala, Gly, or other neutral spacers of nonpolar amino acids or neutral polar amino acids.
- the optionally present spacer need not be comprised of the same residues and thus may be a hetero- or homo-oligomer.
- the spacer will usually be at least one or two residues, more usually three to six residues.
- the CTL peptide may be linked to the T helper peptide without a spacer.
- the immunogenic peptide may be linked to the T helper peptide either directly or via a spacer either at the amino or carboxy terminus of the CTL peptide.
- the amino terminus of either the immunogenic peptide or the T helper peptide may be acylated.
- T helper peptides include tetanus toxoid 830-843, influenza 307-319, malaria circumsporozoite 382-398 and 378-389.
- lipids have been identified as agents capable of priming CTL in vivo against viral antigens.
- palmitic acid residues can be attached to the alpha and epsilon amino groups of a Lys residue and then linked, e.g., via one or more linking residues such as Gly, Gly-Gly-, Ser, Ser-Ser, or the like, to an immunogenic peptide.
- the lipidated peptide can then be injected directly in a micellar form, incorporated into a liposome or emulsified in an adjuvant, e.g. , incomplete Freund's adjuvant.
- a particularly effective immunogen comprises palmitic acid attached to alpha and epsilon amino groups 17 of Lys, which is attached via linkage, e.g. , Ser-Ser, to the amino terminus of the immunogenic peptide.
- E. coli lipoproteins such as tripalmitoyl-S-glycerylcysteinlyseryl-serine (P 3 CSS) can be used to prime virus specific CTL when covalently attached to an appropriate peptide. See, Deres et al. ,
- Peptides of the invention can be coupled to P 3 CSS, for example, and the lipopeptide administered to an individual to specifically prime a CTL response to the target antigen. Further, as the induction of neutralizing antibodies can also be primed with P 3 CSS conjugated to a peptide which displays an appropriate epitope, the two compositions can be combined to more effectively elicit both humoral and cell-mediated responses to infection.
- amino acids can be added to the termini of a peptide to provide for ease of linking peptides one to another, for coupling to a carrier support, or larger peptide, for modifying the physical or chemical properties of the peptide or oligopeptide, or the like.
- Amino acids such as tyrosine, cysteine, lysine, glutamic or aspartic acid, or the like, can be introduced at the C- or N-terminus of the peptide or oligopeptide. Modification at the C terminus in some cases may alter binding characteristics of the peptide.
- the peptide or oligopeptide sequences can differ from the natural sequence by being modified by terminal-NH 2 acylation, e.g.
- alkanoyl C,-C 2o
- thioglycolyl acetylation terminal-carboxyl amidation, e.g. , ammonia, methylamine, etc.
- these modifications may provide sites for linking to a support or other molecule.
- the peptides of the invention can be prepared in a wide variety of ways. Because of their relatively short size, the peptides can be synthesized in solution or on a solid support in accordance with conventional techniques. Various automatic synthesizers are commercially available and can be used in accordance with known protocols. See, for example, Stewart and Young, Solid Phase Peptide Synthesis. 2d. ed. , Pierce Chemical Co. (1984), supra.
- recombinant DNA technology may be employed wherein a nucleotide sequence which encodes an immunogenic peptide of interest is inserted into an expression vector, transformed or transfected into an appropriate host cell and cultivated under conditions suitable for expression.
- a nucleotide sequence which encodes an immunogenic peptide of interest is inserted into an expression vector, transformed or transfected into an appropriate host cell and cultivated under conditions suitable for expression.
- These procedures are generally known in the art, 18 as described generally in Sambrook et al. , Molecular Cloning. A Laboratory Manual. Cold Spring Harbor Press, Cold Spring Harbor, New York (1982), which is incorporated herein by reference.
- fusion proteins which comprise one or more peptide sequences of the invention can be used to present the appropriate T cell epitope.
- coding sequence for peptides of the length contemplated herein can be synthesized by chemical techniques, for example, the phosphotriester method of Matteucci et al. , J. Am. Chem. Soc. 103:3185 (1981), modification can be made simply by substituting the appropriate base(s) for those encoding the native peptide sequence.
- the coding sequence can then be provided with appropriate linkers and ligated into expression vectors commonly available in the art, and the vectors used to transform suitable hosts to produce the desired fusion protein. A number of such vectors and suitable host systems are now available.
- the coding sequence will be provided with operably linked start and stop codons, promoter and terminator regions and usually a replication system to provide an expression vector for expression in the desired cellular host.
- promoter sequences compatible with bacterial hosts are provided in plasmids containing convenient restriction sites for insertion of the desired coding sequence.
- the resulting expression vectors are transformed into suitable bacterial hosts.
- yeast or mammalian cell hosts may also be used, employing suitable vectors and control sequences.
- the peptides of the present invention and pharmaceutical and vaccine compositions thereof are useful for administration to mammals, particularly humans, to treat and/or prevent viral infection and cancer.
- diseases which can be treated using the immunogenic peptides of the invention include prostate cancer, hepatitis B, hepatitis C, AIDS, renal carcinoma, cervical carcinoma, lymphoma, CMV and condlyloma acuminatum.
- the immunogenic peptides of the invention are administered to an individual already suffering from cancer or infected with the virus of interest. Those in the incubation phase or the acute phase of infection can be treated with the immunogenic peptides separately or in conjunction with other treatments, as appropriate.
- compositions are administered to a patient in an amount sufficient to elicit an effective CTL response to the virus or tumor antigen and to cure or at least partially arrest symptoms and/or complications. An amount adequate to 19 accomplish this is defined as " therapeutical ly effective dose. " Amounts effective for this use will depend on, e.g.
- the peptide composition may generally be employed in serious disease states, that is, life- threatening or potentially life threatening situations.
- compositions of the invention may haveten resolution of the infection in acutely infected individuals. For those individuals susceptible (or predisposed) to developing chronic infection the compositions are particularly useful in methods for preventing the evolution from acute to chronic infection. Where the susceptible individuals are identified prior to or during infection, for instance, as described herein, the composition can be targeted to them, minimizing need for administration to a larger population.
- the peptide compositions can also be used for the treatment of chronic infection and to stimulate the immune system to eliminate virus-infected cells in carriers. It is important to provide an amount of immuno-potentiating peptide in a formulation and mode of administration sufficient to effectively stimulate a cytotoxic T cell response.
- a representative dose is in the range of about 1.0 ⁇ g to about 5000 ⁇ g, preferably about 5 ⁇ g to 1000 ⁇ g for a 70 kg patient per dose. 20
- administration should continue until at least clinical symptoms or laboratory tests indicate that the viral infection has been eliminated or substantially abated and for a period thereafter.
- compositions for therapeutic treatment are intended for parenteral, topical, oral or local administration.
- the pharmaceutical compositions are administered parenterally, e.g. , intravenously, subcutaneously, intradermally, or intramuscularly.
- the invention provides compositions for parenteral administration which comprise a solution of the immunogenic peptides dissolved or suspended in an acceptable carrier, preferably an aqueous carrier.
- an acceptable carrier preferably an aqueous carrier.
- aqueous carriers may be used, e.g., water, buffered water, 0.8% saline, 0.3% glycine, hyaluronic acid and the like.
- These compositions may be sterilized by conventional, well known sterilization techniques, or may be sterile filtered.
- compositions may be packaged for use as is, or lyophilized, the lyophilized preparation being combined with a sterile solution prior to administration.
- the compositions may contain pharmaceutically acceptable auxiliary substances as required to approximate physiological conditions, such as pH adjusting and buffering agents, tonicity adjusting agents, wetting agents and the like, for example, sodium acetate, sodium lactate, sodium chloride, potassium chloride, calcium chloride, sorbitan monolaurate, triethanolamine oleate, etc.
- concentration of CTL stimulatory peptides of the invention in the pharmaceutical formulations can vary widely, i.e. , from less than about 0.1 %, usually at or at least about 2% to as much as 20% to 50% or more by weight, and will be selected primarily by fluid volumes, viscosities, etc., in accordance with the particular mode of administration selected.
- the peptides of the invention may also be administered via liposomes, which serve to target the peptides to a particular tissue, such as lymphoid tissue, or targeted selectively to infected cells, as well as increase the half-life of the peptide composition.
- liposomes include emulsions, foams, micelles, insoluble monolayers, liquid crystals, phospholipid dispersions, lamellar layers and the like. In these preparations the peptide to be delivered is incorporated as part of a liposome, alone or in conjunction with a molecule which binds to, e.g.
- liposomes either filled or decorated with a desired peptide of the invention can be directed to the site of lymphoid cells, where the liposomes then deliver the selected therapeutic/immunogenic peptide compositions.
- Liposomes for use in the invention are formed from standard vesicle-forming lipids, which generally include neutral and negatively charged phospholipids and a sterol, such as cholesterol. The selection of lipids is generally guided by consideration of, e.g. , liposome size, acid lability and stability of the liposomes in the blood stream.
- a ligand to be incorporated into the liposome can include, e.g. , antibodies or fragments thereof specific for cell surface determinants of the desired immune system cells.
- a liposome suspension containing a peptide may be administered intravenously, locally, topically, etc. in a dose which varies according to, inter alia, the manner of administration, the peptide being delivered, and the stage of the disease being treated.
- nontoxic solid carriers may be used which include, for example, pharmaceutical grades of mannitol, lactose, starch, magnesium stearate, sodium saccharin, talcum, cellulose, glucose, sucrose, magnesium carbonate, and the like.
- a pharmaceutically acceptable nontoxic composition is formed by incorporating any of the normally employed excipients, such as those carriers previously listed, and generally 10-95% of active ingredient, that is, one or more peptides of the invention, and more preferably at a concentration of 25% -75 % .
- the immunogenic peptides are preferably supplied in finely divided form along with a surfactant and propellant.
- Typical percentages of peptides are 0.01 %-20% by weight, preferably 1 %-10%.
- the surfactant must, of course, be nontoxic, and preferably soluble in the propellant.
- Representative of such agents are the esters or partial esters of fatty acids containing from 6 to 22 carbon atoms, such as caproic, octanoic, lauric, palmitic, stearic, linoleic, linolenic, olesteric and oleic acids with an aliphatic polyhydric alcohol or its cyclic anhydride.
- Mixed esters such as mixed or natural glycerides may be employed.
- the surfactant may constitute 0.1 % -20% by weight 22 of the composition, preferably 0.25-5%.
- the balance of the composition is ordinarily propellant.
- a carrier can also be included, as desired, as with, e.g. , lecithin for intranasal delivery.
- the present invention is directed to vaccines which contain as an active ingredient an immunogenically effective amount of an immunogenic peptide as described herein.
- the peptide(s) may be introduced into a host, including humans, linked to its own carrier or as a homopolymer or heteropolymer of active peptide units.
- a polymer has the advantage of increased immunological reaction and, where different peptides are used to make up the polymer, the additional ability to induce antibodies and/or CTLs that react with different antigenic determinants of the virus or tumor cells.
- Useful carriers are well known in the art, and include, e.g.
- the vaccines can also contain a physiologically tolerable (acceptable) diluent such as water, phosphate buffered saline, or saline, and further typically include an adjuvant.
- Adjuvants such as incomplete Freund's adjuvant, aluminum phosphate, aluminum hydroxide, or alum are materials well known in the art.
- CTL responses can be primed by conjugating peptides of the invention to lipids, such as P 3 CSS.
- lipids such as P 3 CSS.
- the immune system of the host responds to the vaccine by producing large amounts of CTLs specific for the desired antigen, and the host becomes at least partially immune to later infection, or resistant to developing chronic infection.
- Vaccine compositions containing the peptides of the invention are administered to a patient susceptible to or otherwise at risk of viral infection or cancer to elicit an immune response against the antigen and thus enhance the patient's own immune response capabilities.
- a patient susceptible to or otherwise at risk of viral infection or cancer to elicit an immune response against the antigen and thus enhance the patient's own immune response capabilities.
- Such an amount is defined to be an "immunogenically effective dose. " In this use, the precise amounts again depend on the patient's state of health and weight, the mode of administration, the nature of the formulation, etc. , but generally range from about 1.0 ⁇ g to about 5000 ⁇ g per 70 kilogram patient, more commonly from about 10 ⁇ g to about 500 ⁇ g mg per 70 kg of body weight. 23
- peptide vaccines of the invention may be desirable to combine with vaccines which induce neutralizing antibody responses to the virus of interest, particularly to viral envelope antigens.
- nucleic acids encoding one or more of the peptides of the invention can also be admisitered to the patient.
- a number of methods are conveniently used to deliver the nucleic acids to the patient.
- the nulceic acid can be delivered directly, as "naked DNA". This approach is described, for instance, in Wolff et. al. , Science 247: 1465-1468 (1990) as well as U.S. Patent Nos. 5,580,859 and 5,589,466.
- the nucleic acids can also be administered using ballistic delivery as described, for instance, in U.S. Patent No. 5,204,253. Particles comprised solely of DNA can be administered. Alternatively, DNA can be adhered to particles, such as gold particles.
- the nucleci acids can also be delivered complexed to cationic compounds, such as cationic lipids.
- cationic compounds such as cationic lipids.
- Lipid-mediated gene delivery methods are described, for instance, in WO 96/18372; WO 93/24640; Mannino and Gould-Fogerite (1988) BioTechniques 6(7): 682-691; Rose U.S. Pat No. 5,279,833; WO 91/06309; and Feigner et al. (1987) Proc. Natl. Acad. Sci. USA 84: 7413-7414.
- the peptides of the invention can also be expressed by attenuated viral hosts, such as vaccinia or fowlpox.
- vaccinia virus as a vector to express nucleotide sequences that encode the peptides of the invention.
- the recombinant vaccinia virus Upon introduction into an acutely or chronically infected host or into a noninfected host, the recombinant vaccinia virus expresses the immunogenic peptide, and thereby elicits a host CTL response.
- Vaccinia vectors and methods useful in immunization protocols are described in, e.g. , U.S. Patent No. 4,722,848, incorporated herein by reference.
- Another vector is BCG (Bacille Calmette Guerin). BCG vectors are described in Stover et al.
- a preferred means of administering nucleic acids encoding the peptides of the invention uses minigene constructs encoding multiple epitopes of the invention. To create a DNA sequence encoding the selected CTL epitopes (minigene) for expression in human cells, the amino acid sequences of the epitopes are reverse translated. A human codon usage table is used to guide the codon choice for each amino acid.
- DNA sequences are directly adjoined, creating a continuous polypeptide sequence.
- additional elements can be incorporated into the minigene design. Examples of amino acid sequence that could be reverse translated and included in the minigene sequence include: helper T lymphocyte epitopes, a leader (signal) sequence, and an endoplasmic reticulum retention signal.
- MHC presentation of CTL epitopes may be improved by including synthetic (e.g. poly-alanine) or naturally-occurring flanking sequences adjacent to the CTL epitopes.
- the minigene sequence is converted to DNA by assembling oligonucleotides that encode the plus and minus strands of the minigene. Overlapping oligonucleotides (30- 100 bases long) are synthesized, phosphorylated, purified and annealed under appropriate conditions using well known techniques, he ends of the oligonucleotides are joined using T4 DNA ligase. This synthetic minigene, encoding the CTL epitope polypeptide, can then cloned into a desired expression vector.
- Standard regulatory sequences well known to those of skill in the art are included in the vector to ensure expression in the target cells.
- Several vector elements are required: a promoter with a down-stream cloning site for minigene insertion; a polyadenylation signal for efficient transcription termination; an E. coli origin of replication; and an E. coli selectable marker (e.g. ampicillin or kanamycin resistance).
- E. coli origin of replication e.g. ampicillin or kanamycin resistance
- Numerous promoters can be used for this purpose, e.g., the human cytomegalovirus (hCMV) promoter. See, U.S. Patent Nos. 5,580,859 and 5,589,466 for other suitable promoter sequences.
- introns are required for efficient gene expression, and one or more synthetic or naturally-occurring introns could be incorporated into the transcribed region of the minigene.
- mRNA stabilization sequences can also be considered for increasing minigene expression.
- immunostimulatory sequences ISSs or CpGs
- a bicistronic expression vector to allow production of the minigene-encoded epitopes and a second protein included to enhance or decrease immunogenicity
- proteins or polypeptides that could beneficially 25 enhance the immune response if co-expressed include cytokines (e.g. , IL2, IL12, GM- CSF), cytokine-inducing molecules (e.g. LeIF) or costimulatory molecules.
- Helper (HTL) epitopes could be joined to intracellular targeting signals and expressed separately from the CTL epitopes. This would allow direction of the HTL epitopes to a cell compartment different than the CTL epitopes.
- the minigene is cloned into the polylinker region downstream of the promoter.
- This plasmid is transformed into an appropriate E. coli strain, and DNA is prepared using standard techniques. The orientation and DNA sequence of the minigene, as well as all other elements included in the vector, are confirmed using restriction mapping and DNA sequence analysis.
- Bacterial cells harboring the correct plasmid can be stored as a master cell bank and a working cell bank.
- Therapeutic quantities of plasmid DNA are produced by fermentation in E. coli, followed by purification. Aliquots from the working cell bank are used to inoculate fermentation medium (such as Terrific Broth), and grown to saturation in shaker flasks or a bioreactor according to well known techniques. Plasmid DNA can be purified using standard bioseparation technologies such as solid phase anion-exchange resins supplied by Quiagen. If required, supercoiled DNA can be isolated from the open circular and linear forms using gel electrophoresis or other methods.
- Purified plasmid DNA can be prepared for injection using a variety of formulations. The simplest of these is reconstitution of lyophilized DNA in sterile phosphate -buffer saline (PBS). A variety of methods have been described, and new techniques may become available. As noted above, nucleic acids are conveniently formulated with cationic lipids. In addition, glycolipids, fusogenic liposomes, peptides and compounds referred to collectively as protective, interactive, non-condensing (PINC) could also be complexed to purified plasmid DNA to influence variables such as stability, intramuscular dispersion, or trafficking to specific organs or cell types.
- PINC protective, interactive, non-condensing
- Target cell sensitization can be used as a functional assay for expression and MHC class I presentation of minigene-encoded CTL epitopes.
- the plasmid DNA is 26 introduced into a mammalian cell line that is suitable as a target for standard CTL chromium release assays. The transfection method used will be dependent on the final formulation. Electroporation can be used for "naked" DNA, whereas cationic lipids allow direct in vitro transfection.
- a plasmid expressing green fluorescent protein (GFP) can be co-transfected to allow enrichment of transfected cells using fluorescence activated cell sorting (FACS). These cells are then chromium-51 labeled and used as target cells for epitope-specific CTL lines. Cytolysis, detected by 51Cr release, indicates production of MHC presentation of minigene-encoded CTL epitopes.
- GFP green fluorescent protein
- In vivo immunogenicity is a second approach for functional testing of minigene DNA formulations.
- Transgenic mice expressing appropriate human MHC molecules are immunized with the DNA product.
- the dose and route of administration are formulation dependent (e.g. IM for DNA in PBS, IP for lipid-complexed DNA).
- Twenty-one days after immunization splenocytes are harvested and restimulated for 1 week in the presence of peptides encoding each epitope being tested.
- These effector cells (CTLs) are assayed for cytolysis of peptide-loaded, chromium-51 labeled target cells using standard techniques. Lysis of target cells sensitized by MHC loading of peptides corresponding to minigene-encoded epitopes demonstrates DNA vaccine function for in vivo induction of CTLs.
- Antigenic peptides may be used to elicit CTL ex vivo, as well.
- the resulting CTL can be used to treat chronic infections (viral or bacterial) or tumors in patients that do not respond to other conventional forms of therapy, or will not respond to a peptide vaccine approach of therapy.
- Ex vivo CTL responses to a particular pathogen are induced by incubating in tissue culture the patient's CTL precursor cells (CTLp) together with a source of antigen-presenting cells (APC) and the appropriate immunogenic peptide. After an appropriate incubation time (typically 1-4 weeks), in which the CTLp are activated and mature and expand into effector CTL, the cells are infused back into the patient, where they will destroy their specific target cell (an infected cell or a tumor cell).
- the peptides may also find use as diagnostic reagents.
- a peptide of the invention may be used to determine the susceptibility of a particular individual to a treatment regimen which employs the peptide or related peptides, and thus may be helpful in modifying an existing treatment protocol or in determining a prognosis for an affected 27 individual.
- the peptides may also be used to predict which individuals will be at substantial risk for developing chronic infection.
- Class I antigen isolation was carried out as described in the related applications, noted above. Namrally processed peptides were then isolated and sequenced as described there. An allele-specific motif and algorithms were determined and quantitative binding assays were carried out.
- VLVTCLGLSY 10 170 1 0.0048 0 0.0013 0.0007
- VIKNYIHCF 9 1 132 3 ⁇ 0.0003 0 w
- PSLREAALR 9 new 296 3 ⁇ 0.0003 0
- PRALAETSY 9 1 new 268 1 ⁇ 0.0018 ⁇ 0.0003 ⁇ 0.0002
- NYKHCFPEI 9 1 new 135 24 4.8
- IFATCLGLSY 10 3 170 1 ⁇ 0.0002 0.0005 0.0004 00
Abstract
Description
Claims
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2000535367A JP2002507397A (en) | 1998-03-13 | 1998-03-13 | HLA binding peptides and uses thereof |
| EP98910404A EP1064022A4 (en) | 1998-03-13 | 1998-03-13 | Hla-binding peptides and their uses |
| CA002323632A CA2323632A1 (en) | 1998-03-13 | 1998-03-13 | Hla-binding peptides and their uses |
| PCT/US1998/005039 WO1999045954A1 (en) | 1998-03-13 | 1998-03-13 | Hla-binding peptides and their uses |
| AU64655/98A AU6465598A (en) | 1998-03-13 | 1998-03-13 | Hla-binding peptides and their uses |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| PCT/US1998/005039 WO1999045954A1 (en) | 1998-03-13 | 1998-03-13 | Hla-binding peptides and their uses |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO1999045954A1 true WO1999045954A1 (en) | 1999-09-16 |
Family
ID=22266589
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US1998/005039 WO1999045954A1 (en) | 1998-03-13 | 1998-03-13 | Hla-binding peptides and their uses |
Country Status (5)
| Country | Link |
|---|---|
| EP (1) | EP1064022A4 (en) |
| JP (1) | JP2002507397A (en) |
| AU (1) | AU6465598A (en) |
| CA (1) | CA2323632A1 (en) |
| WO (1) | WO1999045954A1 (en) |
Cited By (68)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2000020581A1 (en) * | 1998-10-05 | 2000-04-13 | Ludwig Institute For Cancer Research | Mage-a3 peptides presented by hla class ii molecules |
| WO2000020445A3 (en) * | 1998-10-02 | 2000-07-13 | Pascal Chaux | Tumor antigens and ctl clones isolated by a novel procedure |
| WO2000052045A2 (en) * | 1999-02-26 | 2000-09-08 | Fondazione Centro San Raffaele Del Monte Tabor | Mage-3 derived immunogenic peptides presented by mhc of class ii and the use thereof |
| WO2000032769A3 (en) * | 1998-11-27 | 2000-10-19 | Ludwig Inst Cancer Res | Mage-10 or mage-8 derived hla-a2.1-binding oligopeptides |
| US6183746B1 (en) | 1997-10-09 | 2001-02-06 | Zycos Inc. | Immunogenic peptides from the HPV E7 protein |
| WO2001032193A1 (en) * | 1999-10-29 | 2001-05-10 | Argonex Pharmaceuticals | Cytotoxic t lymphocyte-stimulating peptides for prevention, treatment, and diagnosis of melanoma |
| WO2001036452A2 (en) * | 1999-11-18 | 2001-05-25 | Epimmune Inc. | Heteroclitic analogs of class i epitodes |
| EP1131461A1 (en) * | 1998-11-13 | 2001-09-12 | Oregon Health Sciences University | N-terminally truncated her-2/neu protein as a cancer prognostic indicator |
| WO2001070772A2 (en) * | 2000-03-23 | 2001-09-27 | Pierre Fabre Medicament | Molecule of pharmaceutical interest comprising at its n-terminal a glutamic acid or a glutamine in the form of an addition salt to an acid |
| WO2001074847A2 (en) * | 2000-03-30 | 2001-10-11 | The Government Of The United States Of America Represented By The Secretary, Department Of Health And Human Services | T-cell epitope of mage-12 and related nucleic acids, vectors, cells, compositions and methods of inducing an immune response to cancer |
| WO2002014503A2 (en) * | 2000-08-14 | 2002-02-21 | Corixa Corporation | Compositions and methods for the therapy and diagnosis of her-2/neu-associated malignancies |
| WO2002020053A1 (en) * | 2000-09-01 | 2002-03-14 | Epimmune Inc. | Hla binding peptides and their uses |
| WO2002026785A2 (en) * | 2000-09-28 | 2002-04-04 | Immusystems Gmbh | Epitopes of virus hepatitis c specifically cd4+ t lymphocytes |
| WO2002042325A2 (en) | 2000-10-31 | 2002-05-30 | Zycos Inc. | Cyp1b1 nucleic acids and methods of use |
| US6407063B1 (en) | 1998-10-02 | 2002-06-18 | Ludwig Institute For Cancer Research | Tumor antigens and CTL clones isolated by a novel procedure |
| EP1235841A1 (en) * | 1999-12-10 | 2002-09-04 | Epimmune Inc. | Inducing cellular immune responses to mage2/3 using peptide and nucleic acid compositions |
| WO2002069691A2 (en) * | 2001-03-01 | 2002-09-12 | The Government Of The United States Of America, As Represented By The Secretary, Department Of Health And Human Services, Centers For Disease Control And Prevention, Technology Transfer Office | Immunogenic hiv peptides for use as reagents and vaccines |
| EP1239866A1 (en) * | 1999-12-10 | 2002-09-18 | Epimmune Inc. | INDUCING CELLULAR IMMUNE RESPONSES TO HER2/neu USING PEPTIDE AND NUCLEIC ACID COMPOSITIONS |
| EP1244465A1 (en) * | 1999-12-21 | 2002-10-02 | Epimmune Inc. | Inducing cellular immune responses to prostate cancer antigens using peptide and nucleic acid compositions |
| EP1246644A1 (en) * | 1999-12-10 | 2002-10-09 | Epimmune Inc. | Inducing cellular immune responses to human papillomavirus using peptide and nucleic acid compositions |
| WO2002081646A2 (en) * | 2001-04-06 | 2002-10-17 | Mannkind Corporation | Epitope sequences |
| WO2003016342A2 (en) * | 2001-08-17 | 2003-02-27 | Aventis Pasteur Limited | Tumor antigens for prevention and/or treatment of cancer |
| WO2003029285A2 (en) * | 2001-09-28 | 2003-04-10 | Institut Pasteur | Identification of cd8 epitopes from hiv-1 proteins for prevention and therapy of hiv infections |
| WO2003050140A1 (en) * | 2001-12-10 | 2003-06-19 | Kyogo Itoh | Tumor antigens |
| US6716809B1 (en) | 1997-09-12 | 2004-04-06 | Ludwig Institute For Cancer Research | Mage-A3 peptides presented by HLA class molecules |
| EP1495322A2 (en) * | 2002-04-05 | 2005-01-12 | Epimmune Inc. | Heteroclitic analogs and related methods |
| EP1237564A4 (en) * | 1999-12-10 | 2005-05-04 | Epimmune Inc | Inducing cellular immune responses to p53 using peptide and nucleic acid compositions |
| EP1531860A2 (en) * | 2001-06-05 | 2005-05-25 | The Government of the United States of America, as represented by the Secretary, Department of Health & Human Services | Peptides for chlamydophila pneumoniae vaccine and diagnosis |
| WO2004067570A3 (en) * | 2003-01-28 | 2005-10-13 | Proscan Rx Pharma | Prostate cancer diagnosis and treatment |
| WO2005037190A3 (en) * | 2003-09-03 | 2005-12-29 | Dendritherapeutics Inc | Multiplex vaccines |
| US7071294B1 (en) * | 1998-12-01 | 2006-07-04 | Green Peptide Co., Ltd. | Tumor antigen protein art-1 and tumor antigen peptide thereof |
| EP1752160A2 (en) * | 2001-04-06 | 2007-02-14 | Mannkind Corporation | Epitope sequences |
| US7232682B2 (en) | 2001-11-07 | 2007-06-19 | Mannkind Corporation | Expression vectors encoding epitopes of target-associated antigens and methods for their design |
| US7247712B2 (en) * | 2000-08-11 | 2007-07-24 | Institute Of Microbiology, Chinese Academy Of Sciences | Complex of hepatitis B virus-specific antigenic peptides associated with heat shock proteins and the application thereof |
| WO2007120834A2 (en) | 2006-04-13 | 2007-10-25 | Peptimmune, Inc. | Methods for designing and synthesizing directed sequence polymer compositions via the directed expansion of epitope permeability |
| US7371845B2 (en) | 2001-05-18 | 2008-05-13 | Ludwig Institute For Cancer Research | MAGE-A3 peptides presented by HLA class II molecules |
| US7462354B2 (en) | 1999-12-28 | 2008-12-09 | Pharmexa Inc. | Method and system for optimizing minigenes and peptides encoded thereby |
| US7488793B2 (en) * | 2003-09-22 | 2009-02-10 | Ludwig Institute For Cancer Research | Isolated peptide which binds to HLA-Cw*07 and uses thereof |
| US7611713B2 (en) | 1993-03-05 | 2009-11-03 | Pharmexa Inc. | Inducing cellular immune responses to hepatitis B virus using peptide compositions |
| US7687455B2 (en) | 2004-04-13 | 2010-03-30 | Immune Targeting Systems Ltd. | Antigen delivery vectors and constructs |
| WO2010086294A2 (en) | 2009-01-28 | 2010-08-05 | Epimmune Inc. | Pan-dr binding polypeptides and uses thereof |
| US7795381B2 (en) | 2001-04-09 | 2010-09-14 | Mayo Foundation For Medical Education And Research | Methods and materials for cancer treatment |
| EP2249857A2 (en) * | 2008-01-18 | 2010-11-17 | Aeras Global TB Vaccine Foundation | Malaria vaccine compositions and constituents which elicit cell mediated immunity |
| EP2295074A1 (en) * | 2001-03-07 | 2011-03-16 | Mannkind Corporation | Anti-neovasculature preparations for treating cancer |
| US20110076297A1 (en) * | 2009-09-30 | 2011-03-31 | Saint Louis University | Peptides for Inducing Heterosubtypic Influenza T Cell Responses |
| US20110256163A1 (en) * | 2006-07-12 | 2011-10-20 | Kosmatopoulos Kostantinos Kostas | Identification, optimization and use of cryptic hla-b7 epitopes for immunotherapy |
| US8263560B2 (en) | 2005-04-01 | 2012-09-11 | University Of Maryland Baltimore | HPV 16 peptide vaccine for head and neck cancer |
| EP2559763A1 (en) * | 2004-04-30 | 2013-02-20 | NEC Corporation | HLA-binding peptides, precursors thereof, DNA fragments and recombinant vectors that code for those peptide sequences |
| WO2013036208A1 (en) * | 2011-09-09 | 2013-03-14 | Agency For Science, Technology And Research | P53 activating peptides |
| EP2586460A1 (en) | 2007-10-16 | 2013-05-01 | Peptimmune, Inc. | Method for designing and preparing vaccines comprising directed sequence polymer composition via the directed expansion of epitopes |
| EP2615104A1 (en) * | 2010-09-08 | 2013-07-17 | Saitama Medical University | Hepatitis c virus liposome vaccine |
| US8538706B2 (en) | 2004-04-21 | 2013-09-17 | Lonza Biologics Plc | Method for affinity scoring of peptide/protein complexes |
| US8642531B2 (en) | 2004-04-13 | 2014-02-04 | Immune Targeting Systems Ltd. | Influenza antigen delivery vectors and constructs |
| US8822182B2 (en) | 2001-05-18 | 2014-09-02 | Mayo Foundation For Medical Education And Research | Chimeric antigen-specific T cell-activating polypeptides |
| CN105037559A (en) * | 2015-08-17 | 2015-11-11 | 北京康爱瑞浩生物科技股份有限公司 | Cytotoxic T lymphocyte for treating or preventing hepatitis B and preparation method of cytotoxic T lymphocyte |
| US9320785B2 (en) * | 2012-01-20 | 2016-04-26 | Fernando Thome Kreutz | Autologous cancer cell vaccine |
| US9340577B2 (en) | 1992-08-07 | 2016-05-17 | Epimmune Inc. | HLA binding motifs and peptides and their uses |
| WO2016131856A1 (en) * | 2015-02-18 | 2016-08-25 | F. Hoffmann-La Roche Ag | Immunoconjugates for specific induction of t cell cytotoxicity against a target cell |
| CN107921111A (en) * | 2015-08-28 | 2018-04-17 | 伊玛提克斯生物技术有限公司 | For the new type of peptides of various cancer immunotherapies, peptide combinations and stent |
| US20180243409A1 (en) * | 2003-12-12 | 2018-08-30 | City Of Hope | SYNTHETIC CONJUGATE OF CpG DNA AND T-HELP/CTL PEPTIDE |
| WO2019046818A1 (en) * | 2017-09-01 | 2019-03-07 | Dana-Farber Cancer Institute, Inc. | Immunogenic peptides specific to bcma and taci antigens for treatment of cancer |
| US10287321B2 (en) | 2011-03-17 | 2019-05-14 | The University Of Birmingham | Re-directed immunotherapy |
| EP3545967A1 (en) * | 2018-03-28 | 2019-10-02 | Deutsches Krebsforschungszentrum Stiftung des Öffentlichen Rechts | Cancer immunization platform |
| WO2020178743A1 (en) * | 2019-03-04 | 2020-09-10 | University Health Network | T cell receptors and methods of use thereof |
| US11111277B2 (en) | 2016-12-28 | 2021-09-07 | Invvax, Inc. | Influenza vaccines |
| US11541107B2 (en) | 2015-08-28 | 2023-01-03 | Immatics Biotechnologies Gmbh | Peptides and T cells for use in immunotherapeutic treatment of various cancers |
| EP3908591A4 (en) * | 2019-01-11 | 2023-01-25 | Agency for Science, Technology and Research | Use of hla-a*11:01-restricted hepatitis b virus (hbv) peptides for identifying hbv-specific cd8+ t cells |
| US11957742B2 (en) | 2015-08-28 | 2024-04-16 | Immatics Biotechnologies Gmbh | Method for treating non-small lung cancer with a population of activated T cells |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2005007694A1 (en) * | 2003-07-16 | 2005-01-27 | Green Peptide Co., Ltd. | HER2/neu PEPTIDE AND THERAPEUTIC USE TEHREOF |
| CA2539789A1 (en) * | 2003-09-22 | 2005-03-31 | Green Peptide Co., Ltd. | Peptide originating in hepatitis c virus |
| JP5176099B2 (en) * | 2004-09-27 | 2013-04-03 | 国立大学法人 東京医科歯科大学 | HLA-A11 restricted Tax anti-tumor epitope |
| WO2007029778A1 (en) * | 2005-09-07 | 2007-03-15 | Nec Corporation | Hla-binding peptide, dna fragment encoding the peptide, and recombinant vector |
| CN102286075A (en) * | 2011-08-01 | 2011-12-21 | 中国人民解放军第三军医大学 | Hepatitis B virus surface antigen immunodominance HLA-A*1101 restricted cytotoxic T lymphocyte (CTL) epitope and use thereof |
| JP5999703B2 (en) * | 2013-01-09 | 2016-09-28 | 国立大学法人 東京医科歯科大学 | HLA-DR1-restricted Tax-specific CD4 + T cell epitope |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1994003205A1 (en) * | 1992-08-07 | 1994-02-17 | Cytel Corporation | Hla binding peptides and their uses |
| US20020168374A1 (en) * | 1992-08-07 | 2002-11-14 | Ralph T. Kubo | Hla binding peptides and their uses |
| EP0914142B9 (en) * | 1996-03-11 | 2012-01-04 | Epimmune Inc. | Peptides with increased binding affinity for at least three hla-a3-like molecules |
| DE69733352T2 (en) * | 1996-03-21 | 2006-04-27 | Epimmune, Inc., San Diego | HLA-A2.1 BINDING PEPTIDES AND ITS USE |
| US6413517B1 (en) * | 1997-01-23 | 2002-07-02 | Epimmune, Inc. | Identification of broadly reactive DR restricted epitopes |
-
1998
- 1998-03-13 EP EP98910404A patent/EP1064022A4/en not_active Withdrawn
- 1998-03-13 AU AU64655/98A patent/AU6465598A/en not_active Abandoned
- 1998-03-13 WO PCT/US1998/005039 patent/WO1999045954A1/en active Application Filing
- 1998-03-13 JP JP2000535367A patent/JP2002507397A/en active Pending
- 1998-03-13 CA CA002323632A patent/CA2323632A1/en not_active Abandoned
Non-Patent Citations (5)
| Title |
|---|
| BRUSS V.: "A SHORT LINEAR SEQUENCE IN THE PRE-S DOMAIN OF THE LARGE HEPATITIS B VIRUS ENVELOPE PROTEIN REQUIRED FOR VIRION FORMATION.", JOURNAL OF VIROLOGY., THE AMERICAN SOCIETY FOR MICROBIOLOGY., US, vol. 71., no. 12., 1 December 1997 (1997-12-01), US, pages 9350 - 9357., XP002912056, ISSN: 0022-538X * |
| ENGELHARD V. H.: "STRUCTURE OF PEPTIDES ASSOCIATED WITH MHC CLASS I MOLECULES.", CURRENT OPINION IN IMMUNOLOGY., ELSEVIER, OXFORD., GB, vol. 06., 1 January 1994 (1994-01-01), GB, pages 13 - 23., XP002912059, ISSN: 0952-7915, DOI: 10.1016/0952-7915(94)90028-0 * |
| PREISLER-ADAMS S., ET AL.: "COMPLETE NUCLEOTIDE SEQUENCE OF A HEPATITIS B VIRUS, SUBTYPE ADW2, AND IDENTIFICATION OF THREE TYPES OF C OPEN READING FRAME.", NUCLEIC ACIDS RESEARCH, INFORMATION RETRIEVAL LTD., GB, vol. 21., no. 09., 1 January 1993 (1993-01-01), GB, pages 2258., XP002912057, ISSN: 0305-1048 * |
| RAMMENSEE H.-G., FALK K., ROETZSCHKE O.: "PEPTIDES NATURALLY PRESENTED BY MHC CLASS I MOLECULES.", ANNUAL REVIEW OF IMMUNOLOGY., ANNUAL REVIEWS INC., US, vol. 11., 1 January 1993 (1993-01-01), US, pages 213 - 244., XP002912058, ISSN: 0732-0582, DOI: 10.1146/annurev.iy.11.040193.001241 * |
| See also references of EP1064022A4 * |
Cited By (164)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9340577B2 (en) | 1992-08-07 | 2016-05-17 | Epimmune Inc. | HLA binding motifs and peptides and their uses |
| US7611713B2 (en) | 1993-03-05 | 2009-11-03 | Pharmexa Inc. | Inducing cellular immune responses to hepatitis B virus using peptide compositions |
| US6291430B1 (en) | 1997-09-12 | 2001-09-18 | Ludwig Institute For Cancer Research | Mage-3 peptides presented by HLA class II molecules |
| US6716809B1 (en) | 1997-09-12 | 2004-04-06 | Ludwig Institute For Cancer Research | Mage-A3 peptides presented by HLA class molecules |
| US6426217B1 (en) | 1997-09-12 | 2002-07-30 | Ludwig Institute For Cancer Research | MAGE-3 peptides presented by HLA class II molecules |
| US6183746B1 (en) | 1997-10-09 | 2001-02-06 | Zycos Inc. | Immunogenic peptides from the HPV E7 protein |
| US7097843B2 (en) | 1997-10-09 | 2006-08-29 | Mgi Pharma Biologics, Inc. | Immunogenic peptides from the HPV E7 protein |
| US6582704B2 (en) | 1997-10-09 | 2003-06-24 | Zycos Inc. | Immunogenic peptides from the HPV E7 protein |
| US7351409B2 (en) | 1998-10-02 | 2008-04-01 | Ludwig Institute For Cancer Research | Tumor antigens and CTL clones isolated by a novel procedure |
| US6407063B1 (en) | 1998-10-02 | 2002-06-18 | Ludwig Institute For Cancer Research | Tumor antigens and CTL clones isolated by a novel procedure |
| US6531451B1 (en) | 1998-10-02 | 2003-03-11 | Ludwig Institute For Cancer Research | Tumor antigens and CTL clones isolated by a novel procedure |
| WO2000020445A3 (en) * | 1998-10-02 | 2000-07-13 | Pascal Chaux | Tumor antigens and ctl clones isolated by a novel procedure |
| WO2000020581A1 (en) * | 1998-10-05 | 2000-04-13 | Ludwig Institute For Cancer Research | Mage-a3 peptides presented by hla class ii molecules |
| EP1131461A1 (en) * | 1998-11-13 | 2001-09-12 | Oregon Health Sciences University | N-terminally truncated her-2/neu protein as a cancer prognostic indicator |
| EP2299272A3 (en) * | 1998-11-13 | 2011-08-03 | Oregon Health Sciences University | N-terminally truncated HER-2/NEU protein as a cancerprognostic indicator |
| EP2299272A2 (en) * | 1998-11-13 | 2011-03-23 | Oregon Health Sciences University | N-terminally truncated HER-2/NEU protein as a cancerprognostic indicator |
| EP2147978A3 (en) * | 1998-11-13 | 2010-07-14 | Oregon Health Sciences University | N-Terminally truncated HER-2/NEU protein as a cancer prognostic indicator |
| EP1131461A4 (en) * | 1998-11-13 | 2005-03-02 | Univ Oregon Health Sciences | N-terminally truncated her-2/neu protein as a cancer prognostic indicator |
| US8093216B2 (en) | 1998-11-13 | 2012-01-10 | Oregon Health & Science University | Method of treating cancer by inhibition of p95HER-2 production |
| US7547439B1 (en) | 1998-11-27 | 2009-06-16 | Ludwig Institute For Cancer Research | Tumor rejection antigens |
| WO2000032769A3 (en) * | 1998-11-27 | 2000-10-19 | Ludwig Inst Cancer Res | Mage-10 or mage-8 derived hla-a2.1-binding oligopeptides |
| US8519109B2 (en) | 1998-11-27 | 2013-08-27 | Ludwig Institute For Cancer Research Ltd. | Tumour rejection antigens |
| US7071294B1 (en) * | 1998-12-01 | 2006-07-04 | Green Peptide Co., Ltd. | Tumor antigen protein art-1 and tumor antigen peptide thereof |
| WO2000052045A3 (en) * | 1999-02-26 | 2001-01-04 | San Raffaele Centro Fond | Mage-3 derived immunogenic peptides presented by mhc of class ii and the use thereof |
| WO2000052045A2 (en) * | 1999-02-26 | 2000-09-08 | Fondazione Centro San Raffaele Del Monte Tabor | Mage-3 derived immunogenic peptides presented by mhc of class ii and the use thereof |
| WO2001032193A1 (en) * | 1999-10-29 | 2001-05-10 | Argonex Pharmaceuticals | Cytotoxic t lymphocyte-stimulating peptides for prevention, treatment, and diagnosis of melanoma |
| US8741576B2 (en) | 1999-11-18 | 2014-06-03 | Epimunne Inc. | Heteroclitic analogs and related methods |
| WO2001036452A2 (en) * | 1999-11-18 | 2001-05-25 | Epimmune Inc. | Heteroclitic analogs of class i epitodes |
| WO2001036452A3 (en) * | 1999-11-18 | 2002-01-10 | Epimmune Inc | Heteroclitic analogs of class i epitodes |
| EP2177534A3 (en) * | 1999-11-18 | 2010-07-14 | Pharmexa Inc. | Heteroclitic analogs of class i epitopes |
| JP4776131B2 (en) * | 1999-11-18 | 2011-09-21 | エピミューン インコーポレイテッド | Heteroclitic analogs and related methods |
| JP2003521243A (en) * | 1999-11-18 | 2003-07-15 | エピミューン, インコーポレイテッド | Heterocritic analogs and related methods |
| JP2004512814A (en) * | 1999-12-10 | 2004-04-30 | エピミューン インコーポレイテッド | Induction of a cellular immune response against human papillomavirus using peptide and nucleic acid compositions |
| EP1235841A1 (en) * | 1999-12-10 | 2002-09-04 | Epimmune Inc. | Inducing cellular immune responses to mage2/3 using peptide and nucleic acid compositions |
| EP1239866A1 (en) * | 1999-12-10 | 2002-09-18 | Epimmune Inc. | INDUCING CELLULAR IMMUNE RESPONSES TO HER2/neu USING PEPTIDE AND NUCLEIC ACID COMPOSITIONS |
| EP1246644A1 (en) * | 1999-12-10 | 2002-10-09 | Epimmune Inc. | Inducing cellular immune responses to human papillomavirus using peptide and nucleic acid compositions |
| EP1235841A4 (en) * | 1999-12-10 | 2006-04-12 | Epimmune Inc | Inducing cellular immune responses to mage2/3 using peptide and nucleic acid compositions |
| US7026443B1 (en) | 1999-12-10 | 2006-04-11 | Epimmune Inc. | Inducing cellular immune responses to human Papillomavirus using peptide and nucleic acid compositions |
| US7572882B2 (en) | 1999-12-10 | 2009-08-11 | Pharmexa Inc. | Inducing cellular immune responses to human papillomavirus using peptide and nucleic acid compositions |
| US20130017253A1 (en) * | 1999-12-10 | 2013-01-17 | Alessandro Sette | Inducing Cellular Immune Responses to Human Papillomavirus Using Peptide and Nucleic Acid Compositions |
| EP1568373A3 (en) * | 1999-12-10 | 2005-12-21 | Epimmune Inc. | Inducing cellular immune responses to HER2/neu using peptide and nucleic acid compositions |
| EP1568373A2 (en) * | 1999-12-10 | 2005-08-31 | Epimmune Inc. | Inducing cellular immune responses to HER2/neu using peptide and nucleic acid compositions |
| EP1246644A4 (en) * | 1999-12-10 | 2005-05-11 | Epimmune Inc | Inducing cellular immune responses to human papillomavirus using peptide and nucleic acid compositions |
| EP1237564A4 (en) * | 1999-12-10 | 2005-05-04 | Epimmune Inc | Inducing cellular immune responses to p53 using peptide and nucleic acid compositions |
| EP1239866A4 (en) * | 1999-12-10 | 2005-02-09 | Epimmune Inc | INDUCING CELLULAR IMMUNE RESPONSES TO HER2/neu USING PEPTIDE AND NUCLEIC ACID COMPOSITIONS |
| EP1244465A4 (en) * | 1999-12-21 | 2005-01-12 | Epimmune Inc | Inducing cellular immune responses to prostate cancer antigens using peptide and nucleic acid compositions |
| EP1244465A1 (en) * | 1999-12-21 | 2002-10-02 | Epimmune Inc. | Inducing cellular immune responses to prostate cancer antigens using peptide and nucleic acid compositions |
| US7462354B2 (en) | 1999-12-28 | 2008-12-09 | Pharmexa Inc. | Method and system for optimizing minigenes and peptides encoded thereby |
| WO2001070772A2 (en) * | 2000-03-23 | 2001-09-27 | Pierre Fabre Medicament | Molecule of pharmaceutical interest comprising at its n-terminal a glutamic acid or a glutamine in the form of an addition salt to an acid |
| FR2806727A1 (en) * | 2000-03-23 | 2001-09-28 | Pf Medicament | MOLECULE OF PHARMACEUTICAL INTEREST COMPRISING IN THE N-TERMINAL END A GLUTAMIC ACID OR GLUTAMINE IN THE FORM OF A PHYSIOLOGICALLY ACCEPTABLE ACID ADDITION SALT |
| WO2001070772A3 (en) * | 2000-03-23 | 2003-02-13 | Pf Medicament | Molecule of pharmaceutical interest comprising at its n-terminal a glutamic acid or a glutamine in the form of an addition salt to an acid |
| WO2001074847A3 (en) * | 2000-03-30 | 2002-03-21 | Us Health | T-cell epitope of mage-12 and related nucleic acids, vectors, cells, compositions and methods of inducing an immune response to cancer |
| WO2001074847A2 (en) * | 2000-03-30 | 2001-10-11 | The Government Of The United States Of America Represented By The Secretary, Department Of Health And Human Services | T-cell epitope of mage-12 and related nucleic acids, vectors, cells, compositions and methods of inducing an immune response to cancer |
| US7247712B2 (en) * | 2000-08-11 | 2007-07-24 | Institute Of Microbiology, Chinese Academy Of Sciences | Complex of hepatitis B virus-specific antigenic peptides associated with heat shock proteins and the application thereof |
| WO2002014503A3 (en) * | 2000-08-14 | 2003-09-18 | Corixa Corp | Compositions and methods for the therapy and diagnosis of her-2/neu-associated malignancies |
| WO2002014503A2 (en) * | 2000-08-14 | 2002-02-21 | Corixa Corporation | Compositions and methods for the therapy and diagnosis of her-2/neu-associated malignancies |
| JP2004522412A (en) * | 2000-08-14 | 2004-07-29 | コリクサ コーポレイション | Compositions and methods for the treatment and diagnosis of HER-2 / NEU-related malignancies |
| WO2002020053A1 (en) * | 2000-09-01 | 2002-03-14 | Epimmune Inc. | Hla binding peptides and their uses |
| EP1195381A1 (en) * | 2000-09-28 | 2002-04-10 | Immusystems GmbH | Hepatitis c virus epitopes specific for cd4+ t-cell lymphocytes |
| WO2002026785A2 (en) * | 2000-09-28 | 2002-04-04 | Immusystems Gmbh | Epitopes of virus hepatitis c specifically cd4+ t lymphocytes |
| WO2002026785A3 (en) * | 2000-09-28 | 2003-11-06 | Immusystems Gmbh | Epitopes of virus hepatitis c specifically cd4+ t lymphocytes |
| US7108856B2 (en) | 2000-09-28 | 2006-09-19 | Immusystems Gmbh | CD4+ T-lymphocyte-specific Hepatitis C virus-epitopes |
| WO2002042325A2 (en) | 2000-10-31 | 2002-05-30 | Zycos Inc. | Cyp1b1 nucleic acids and methods of use |
| EP2295578A2 (en) | 2000-10-31 | 2011-03-16 | Eisai Inc. | Cyp1b1 nucleic acids and methods of use |
| WO2002069691A2 (en) * | 2001-03-01 | 2002-09-12 | The Government Of The United States Of America, As Represented By The Secretary, Department Of Health And Human Services, Centers For Disease Control And Prevention, Technology Transfer Office | Immunogenic hiv peptides for use as reagents and vaccines |
| EP1490396A4 (en) * | 2001-03-01 | 2006-04-19 | Us Gov Health & Human Serv | Immunogenic hiv peptides for use as reagents and vaccines |
| WO2002069691A3 (en) * | 2001-03-01 | 2004-09-16 | Us Gov Health & Human Serv | Immunogenic hiv peptides for use as reagents and vaccines |
| EP2295074A1 (en) * | 2001-03-07 | 2011-03-16 | Mannkind Corporation | Anti-neovasculature preparations for treating cancer |
| EP2394655A2 (en) * | 2001-04-06 | 2011-12-14 | Mannkind Corporation | Epitope sequences |
| EP2394655A3 (en) * | 2001-04-06 | 2012-05-02 | Mannkind Corporation | Epitope sequences |
| JP2005509404A (en) * | 2001-04-06 | 2005-04-14 | マンカインド コーポレイション | Epitope sequence |
| WO2002081646A2 (en) * | 2001-04-06 | 2002-10-17 | Mannkind Corporation | Epitope sequences |
| EP1752160A2 (en) * | 2001-04-06 | 2007-02-14 | Mannkind Corporation | Epitope sequences |
| WO2002081646A3 (en) * | 2001-04-06 | 2003-07-17 | Mannkind Corp | Epitope sequences |
| JP2009060910A (en) * | 2001-04-06 | 2009-03-26 | Mannkind Corp | Epitope sequences |
| JP4874508B2 (en) * | 2001-04-06 | 2012-02-15 | マンカインド コーポレイション | Epitope sequence |
| US7795381B2 (en) | 2001-04-09 | 2010-09-14 | Mayo Foundation For Medical Education And Research | Methods and materials for cancer treatment |
| US8822182B2 (en) | 2001-05-18 | 2014-09-02 | Mayo Foundation For Medical Education And Research | Chimeric antigen-specific T cell-activating polypeptides |
| US7371845B2 (en) | 2001-05-18 | 2008-05-13 | Ludwig Institute For Cancer Research | MAGE-A3 peptides presented by HLA class II molecules |
| US7388072B2 (en) | 2001-05-18 | 2008-06-17 | Ludwig Institute For Cancer Research | MAGE peptides presented by HLA class II molecules |
| EP1531860A4 (en) * | 2001-06-05 | 2005-11-02 | Us Gov Health & Human Serv | Peptides for chlamydophila pneumoniae vaccine and diagnosis |
| EP1531860A2 (en) * | 2001-06-05 | 2005-05-25 | The Government of the United States of America, as represented by the Secretary, Department of Health & Human Services | Peptides for chlamydophila pneumoniae vaccine and diagnosis |
| US7223836B2 (en) | 2001-06-05 | 2007-05-29 | The United States Of America As Represented By The Secretary Of The Department Of Health And Human Services, Centers For Disease Control And Prevention | Peptides for chlamydophila pneumoniae vaccine and diagnosis |
| WO2003016342A3 (en) * | 2001-08-17 | 2003-11-27 | Aventis Pasteur | Tumor antigens for prevention and/or treatment of cancer |
| WO2003016342A2 (en) * | 2001-08-17 | 2003-02-27 | Aventis Pasteur Limited | Tumor antigens for prevention and/or treatment of cancer |
| WO2003029285A3 (en) * | 2001-09-28 | 2004-01-22 | Pasteur Institut | Identification of cd8 epitopes from hiv-1 proteins for prevention and therapy of hiv infections |
| US7022325B2 (en) | 2001-09-28 | 2006-04-04 | Institut Pasteur | CD8 epitope from HIV-1 protein Vpu |
| US7431928B2 (en) | 2001-09-28 | 2008-10-07 | Institut Pasteur | Identification of new CD8 epitopes from HIV-1 proteins |
| WO2003029285A2 (en) * | 2001-09-28 | 2003-04-10 | Institut Pasteur | Identification of cd8 epitopes from hiv-1 proteins for prevention and therapy of hiv infections |
| US8637305B2 (en) | 2001-11-07 | 2014-01-28 | Mannkind Corporation | Expression vectors encoding epitopes of target-associated antigens and methods for their design |
| US8252916B2 (en) * | 2001-11-07 | 2012-08-28 | Mannkind Corporation | Expression vectors encoding epitopes of target-associated antigens and methods for their design |
| US7232682B2 (en) | 2001-11-07 | 2007-06-19 | Mannkind Corporation | Expression vectors encoding epitopes of target-associated antigens and methods for their design |
| WO2003050140A1 (en) * | 2001-12-10 | 2003-06-19 | Kyogo Itoh | Tumor antigens |
| EP1495322A2 (en) * | 2002-04-05 | 2005-01-12 | Epimmune Inc. | Heteroclitic analogs and related methods |
| EP1495322A4 (en) * | 2002-04-05 | 2006-09-06 | Epimmune Inc | Heteroclitic analogs and related methods |
| US7811564B2 (en) | 2003-01-28 | 2010-10-12 | Proscan Rx Pharma | Prostate cancer diagnosis and treatment |
| US8101713B2 (en) | 2003-01-28 | 2012-01-24 | Proscan Rx Pharma Inc. | Prostate cancer diagnosis and treatment |
| EP1587837A3 (en) * | 2003-01-28 | 2005-12-07 | Proscan RX Pharma | Prostate cancer diagnosis and treatment |
| WO2004067570A3 (en) * | 2003-01-28 | 2005-10-13 | Proscan Rx Pharma | Prostate cancer diagnosis and treatment |
| US8512702B2 (en) | 2003-01-28 | 2013-08-20 | Proscan Rx Pharma Inc. | Prostate cancer diagnosis and treatment |
| JP2007504237A (en) * | 2003-09-03 | 2007-03-01 | デンドリセラピューティクス、インク. | Combined vaccine |
| WO2005037190A3 (en) * | 2003-09-03 | 2005-12-29 | Dendritherapeutics Inc | Multiplex vaccines |
| AU2004281634B2 (en) * | 2003-09-03 | 2011-01-27 | Dendritherapeutics, Inc. | Multiplex vaccines |
| US7488793B2 (en) * | 2003-09-22 | 2009-02-10 | Ludwig Institute For Cancer Research | Isolated peptide which binds to HLA-Cw*07 and uses thereof |
| US20180243409A1 (en) * | 2003-12-12 | 2018-08-30 | City Of Hope | SYNTHETIC CONJUGATE OF CpG DNA AND T-HELP/CTL PEPTIDE |
| US10596254B2 (en) * | 2003-12-12 | 2020-03-24 | City Of Hope | Synthetic conjugate of CpG DNA and T-help/CTL peptide |
| US10987420B2 (en) | 2003-12-12 | 2021-04-27 | City Of Hope | Synthetic conjugate of CpG DNA and T-help/CTL peptide |
| US8642531B2 (en) | 2004-04-13 | 2014-02-04 | Immune Targeting Systems Ltd. | Influenza antigen delivery vectors and constructs |
| US8759281B2 (en) | 2004-04-13 | 2014-06-24 | Immune Targeting Systems Ltd. | Antigen delivery vectors and constructs |
| US11066353B2 (en) | 2004-04-13 | 2021-07-20 | Altimmune Uk Ltd | Antigen delivery vectors and constructs |
| US7687455B2 (en) | 2004-04-13 | 2010-03-30 | Immune Targeting Systems Ltd. | Antigen delivery vectors and constructs |
| US8129333B2 (en) | 2004-04-13 | 2012-03-06 | Immune Targeting Systems Ltd. | Antigen delivery vectors and constructs |
| US8110540B2 (en) | 2004-04-13 | 2012-02-07 | Immune Targeting Systems Ltd. | Antigen delivery vectors and constructs |
| US8538706B2 (en) | 2004-04-21 | 2013-09-17 | Lonza Biologics Plc | Method for affinity scoring of peptide/protein complexes |
| EP2559763A1 (en) * | 2004-04-30 | 2013-02-20 | NEC Corporation | HLA-binding peptides, precursors thereof, DNA fragments and recombinant vectors that code for those peptide sequences |
| US8263560B2 (en) | 2005-04-01 | 2012-09-11 | University Of Maryland Baltimore | HPV 16 peptide vaccine for head and neck cancer |
| WO2007120834A2 (en) | 2006-04-13 | 2007-10-25 | Peptimmune, Inc. | Methods for designing and synthesizing directed sequence polymer compositions via the directed expansion of epitope permeability |
| KR101454935B1 (en) * | 2006-07-12 | 2014-10-28 | 바쏭 비오떼끄 | Identification, optimization and use of cryptic hla-b7 epitopes for immunotherapy |
| US20110256163A1 (en) * | 2006-07-12 | 2011-10-20 | Kosmatopoulos Kostantinos Kostas | Identification, optimization and use of cryptic hla-b7 epitopes for immunotherapy |
| US8465747B2 (en) * | 2006-07-12 | 2013-06-18 | Vaxon Biotech | Identification, optimization and use of cryptic HLA-B7 epitopes for immunotherapy |
| US20170202936A1 (en) * | 2006-07-12 | 2017-07-20 | Vaxon Biotech | Identification, Optimization And Use Of Cryptic HLA-B7 Epitopes For Immunotherapy |
| US10155049B2 (en) | 2007-08-31 | 2018-12-18 | Altimmune UK, LTD | Influenza antigen delivery vectors and constructs |
| US9446143B2 (en) | 2007-08-31 | 2016-09-20 | Altimmune Uk Limited | Influenza antigen delivery vectors and constructs |
| EP2586460A1 (en) | 2007-10-16 | 2013-05-01 | Peptimmune, Inc. | Method for designing and preparing vaccines comprising directed sequence polymer composition via the directed expansion of epitopes |
| US8728492B2 (en) | 2008-01-18 | 2014-05-20 | Aeras Global Tb Vaccine Foundation | Malaria vaccine compositions and constituents which elicit cell mediated immunity |
| EP2249857A4 (en) * | 2008-01-18 | 2012-02-29 | Aeras Global Tb Vaccine Foundation | Malaria vaccine compositions and constituents which elicit cell mediated immunity |
| EP2249857A2 (en) * | 2008-01-18 | 2010-11-17 | Aeras Global TB Vaccine Foundation | Malaria vaccine compositions and constituents which elicit cell mediated immunity |
| WO2010086294A2 (en) | 2009-01-28 | 2010-08-05 | Epimmune Inc. | Pan-dr binding polypeptides and uses thereof |
| US9265822B2 (en) * | 2009-09-30 | 2016-02-23 | Saint Louis University | Peptides for inducing heterosubtypic influenza T cell responses |
| US20110076297A1 (en) * | 2009-09-30 | 2011-03-31 | Saint Louis University | Peptides for Inducing Heterosubtypic Influenza T Cell Responses |
| EP2615104A4 (en) * | 2010-09-08 | 2014-03-19 | Univ Saitama Medical | Hepatitis c virus liposome vaccine |
| EP2615104A1 (en) * | 2010-09-08 | 2013-07-17 | Saitama Medical University | Hepatitis c virus liposome vaccine |
| US9814785B2 (en) | 2010-09-08 | 2017-11-14 | Saitama Medical University | Hepatitis C virus liposome vaccine |
| US10287321B2 (en) | 2011-03-17 | 2019-05-14 | The University Of Birmingham | Re-directed immunotherapy |
| US11236131B2 (en) | 2011-03-17 | 2022-02-01 | The University Of Birmingham | Re-directed immunotherapy |
| US9517252B2 (en) | 2011-09-09 | 2016-12-13 | Agency For Science, Technology And Research | p53 activating peptides |
| WO2013036208A1 (en) * | 2011-09-09 | 2013-03-14 | Agency For Science, Technology And Research | P53 activating peptides |
| US9320785B2 (en) * | 2012-01-20 | 2016-04-26 | Fernando Thome Kreutz | Autologous cancer cell vaccine |
| WO2016131856A1 (en) * | 2015-02-18 | 2016-08-25 | F. Hoffmann-La Roche Ag | Immunoconjugates for specific induction of t cell cytotoxicity against a target cell |
| RU2746021C2 (en) * | 2015-02-18 | 2021-04-06 | Ф.Хоффманн-Ля Рош Аг | Immunoconjugates for the specific induction of t cell cytotoxicity against target cells |
| CN105037559A (en) * | 2015-08-17 | 2015-11-11 | 北京康爱瑞浩生物科技股份有限公司 | Cytotoxic T lymphocyte for treating or preventing hepatitis B and preparation method of cytotoxic T lymphocyte |
| CN105037559B (en) * | 2015-08-17 | 2018-09-25 | 北京康爱瑞浩生物科技股份有限公司 | Treat or prevent the cytotoxic T lymphocyte and preparation method thereof of hepatitis B |
| US11744882B2 (en) | 2015-08-28 | 2023-09-05 | Immatics Biotechnologies Gmbh | Peptides and T cells for use in immunotherapeutic treatment of various cancers |
| US11957742B2 (en) | 2015-08-28 | 2024-04-16 | Immatics Biotechnologies Gmbh | Method for treating non-small lung cancer with a population of activated T cells |
| US10898558B2 (en) | 2015-08-28 | 2021-01-26 | Immatics Biotechnologies Gmbh | Method of treating with a peptide |
| US10898557B2 (en) | 2015-08-28 | 2021-01-26 | Immatics Biotechnologies Gmbh | Method of treating with a peptide |
| US11793866B2 (en) | 2015-08-28 | 2023-10-24 | Immatics Biotechnologies Gmbh | Peptides and T cells for use in immunotherapeutic treatment of various cancers |
| US11951160B2 (en) | 2015-08-28 | 2024-04-09 | Immatics Biotechnologies Gmbh | Peptides and T cells for use in immunotherapeutic treatment of various cancers |
| US11065316B2 (en) | 2015-08-28 | 2021-07-20 | Immatics Biotechnologies Gmbh | Peptides and T cells for use in immunother[[r]]apeutic treatment of various cancers |
| US11576954B2 (en) | 2015-08-28 | 2023-02-14 | Immatics Biotechnologies Gmbh | Method for treating non-small lung cancer with a population of activated cells |
| US11559572B2 (en) | 2015-08-28 | 2023-01-24 | Immatics Biotechnologies Gmbh | Peptides and T cells for use in immunotherapeutic treatment of various cancers |
| CN107921111A (en) * | 2015-08-28 | 2018-04-17 | 伊玛提克斯生物技术有限公司 | For the new type of peptides of various cancer immunotherapies, peptide combinations and stent |
| CN107921111B (en) * | 2015-08-28 | 2022-07-26 | 伊玛提克斯生物技术有限公司 | Novel peptides, peptide compositions and scaffolds for immunotherapy of various cancers |
| US11547750B2 (en) | 2015-08-28 | 2023-01-10 | Immatics Biotechnologies Gmbh | Peptides and T cells for use in immunotherapeutic treatment hepatocellular carcinoma |
| US11541107B2 (en) | 2015-08-28 | 2023-01-03 | Immatics Biotechnologies Gmbh | Peptides and T cells for use in immunotherapeutic treatment of various cancers |
| US11739127B2 (en) | 2016-12-28 | 2023-08-29 | Invvax, Inc. | Influenza vaccines |
| US11111277B2 (en) | 2016-12-28 | 2021-09-07 | Invvax, Inc. | Influenza vaccines |
| WO2019046818A1 (en) * | 2017-09-01 | 2019-03-07 | Dana-Farber Cancer Institute, Inc. | Immunogenic peptides specific to bcma and taci antigens for treatment of cancer |
| US11517591B2 (en) | 2017-09-01 | 2022-12-06 | Dana-Farber Cancer Institute, Inc. | Immunogenic peptides specific to BCMA and TACI antigens |
| JP2020532300A (en) * | 2017-09-01 | 2020-11-12 | デイナ ファーバー キャンサー インスティチュート,インコーポレイテッド | Immunogenic peptides specific for BCMA and TACI antigens for the treatment of cancer |
| CN111655280A (en) * | 2017-09-01 | 2020-09-11 | 达纳-法伯癌症研究所股份有限公司 | BCMA and TACI antigen specific immunogenic peptides for the treatment of cancer |
| EP3545967A1 (en) * | 2018-03-28 | 2019-10-02 | Deutsches Krebsforschungszentrum Stiftung des Öffentlichen Rechts | Cancer immunization platform |
| EP3908591A4 (en) * | 2019-01-11 | 2023-01-25 | Agency for Science, Technology and Research | Use of hla-a*11:01-restricted hepatitis b virus (hbv) peptides for identifying hbv-specific cd8+ t cells |
| WO2020178743A1 (en) * | 2019-03-04 | 2020-09-10 | University Health Network | T cell receptors and methods of use thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| AU6465598A (en) | 1999-09-27 |
| JP2002507397A (en) | 2002-03-12 |
| EP1064022A4 (en) | 2004-09-29 |
| EP1064022A1 (en) | 2001-01-03 |
| CA2323632A1 (en) | 1999-09-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP1064022A1 (en) | Hla-binding peptides and their uses | |
| US7252829B1 (en) | HLA binding peptides and their uses | |
| EP1917970B1 (en) | Hla binding peptides and their uses | |
| AU725550B2 (en) | HLA binding peptides and their uses | |
| CA2248667C (en) | Hla-a2.1 binding peptides and their uses | |
| WO2004031211A2 (en) | Hla binding peptides and their uses | |
| WO2001062776A1 (en) | Hla binding peptides and their uses | |
| US20020177694A1 (en) | Hla binding peptides and their uses | |
| EP1089757B1 (en) | Hla binding peptides and their uses | |
| EP1343819A1 (en) | Hla-a2.1 binding peptides and their uses | |
| EP1320377B1 (en) | Hla binding peptides and their uses | |
| EP1767542B1 (en) | HLA-A2.1 binding peptides and their uses | |
| WO2002020053A1 (en) | Hla binding peptides and their uses |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AL AM AT AU AZ BA BB BG BR BY CA CH CN CU CZ DE DK EE ES FI GB GE GH GM GW HU ID IL IS JP KE KG KP KR KZ LC LK LR LS LT LU LV MD MG MK MN MW MX NO NZ PL PT RO RU SD SE SG SI SK SL TJ TM TR TT UA UG UZ VN YU ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): GH GM KE LS MW SD SZ UG ZW AM AZ BY KG KZ MD RU TJ TM AT BE CH DE DK ES FI FR GB GR IE IT LU MC NL PT SE BF BJ CF CG CI CM GA GN ML MR NE SN TD TG |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| DFPE | Request for preliminary examination filed prior to expiration of 19th month from priority date (pct application filed before 20040101) | ||
| ENP | Entry into the national phase |
Ref document number: 2323632 Country of ref document: CA Ref country code: CA Ref document number: 2323632 Kind code of ref document: A Format of ref document f/p: F |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 64655/98 Country of ref document: AU |
|
| NENP | Non-entry into the national phase |
Ref country code: KR |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1998910404 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 507463 Country of ref document: NZ |
|
| WWP | Wipo information: published in national office |
Ref document number: 1998910404 Country of ref document: EP |
|
| REG | Reference to national code |
Ref country code: DE Ref legal event code: 8642 |