WO1998044079A1 - Lubricating compositions - Google Patents
Lubricating compositions Download PDFInfo
- Publication number
- WO1998044079A1 WO1998044079A1 PCT/EP1998/001899 EP9801899W WO9844079A1 WO 1998044079 A1 WO1998044079 A1 WO 1998044079A1 EP 9801899 W EP9801899 W EP 9801899W WO 9844079 A1 WO9844079 A1 WO 9844079A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- groups
- mole
- oil composition
- composition according
- alkyl groups
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M163/00—Lubricating compositions characterised by the additive being a mixture of a compound of unknown or incompletely defined constitution and a non-macromolecular compound, each of these compounds being essential
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M129/00—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing oxygen
- C10M129/02—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing oxygen having a carbon chain of less than 30 atoms
- C10M129/04—Hydroxy compounds
- C10M129/10—Hydroxy compounds having hydroxy groups bound to a carbon atom of a six-membered aromatic ring
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M129/00—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing oxygen
- C10M129/02—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing oxygen having a carbon chain of less than 30 atoms
- C10M129/26—Carboxylic acids; Salts thereof
- C10M129/48—Carboxylic acids; Salts thereof having carboxyl groups bound to a carbon atom of a six-membered aromatic ring
- C10M129/54—Carboxylic acids; Salts thereof having carboxyl groups bound to a carbon atom of a six-membered aromatic ring containing hydroxy groups
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M133/00—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing nitrogen
- C10M133/02—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing nitrogen having a carbon chain of less than 30 atoms
- C10M133/04—Amines, e.g. polyalkylene polyamines; Quaternary amines
- C10M133/12—Amines, e.g. polyalkylene polyamines; Quaternary amines having amino groups bound to a carbon atom of a six-membered aromatic ring
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M133/00—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing nitrogen
- C10M133/52—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing nitrogen having a carbon chain of 30 or more atoms
- C10M133/56—Amides; Imides
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M135/00—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing sulfur, selenium or tellurium
- C10M135/08—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing sulfur, selenium or tellurium containing a sulfur-to-oxygen bond
- C10M135/10—Sulfonic acids or derivatives thereof
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M135/00—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing sulfur, selenium or tellurium
- C10M135/20—Thiols; Sulfides; Polysulfides
- C10M135/28—Thiols; Sulfides; Polysulfides containing sulfur atoms bound to a carbon atom of a six-membered aromatic ring
- C10M135/30—Thiols; Sulfides; Polysulfides containing sulfur atoms bound to a carbon atom of a six-membered aromatic ring containing hydroxy groups; Derivatives thereof
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M137/00—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing phosphorus
- C10M137/02—Lubricating compositions characterised by the additive being an organic non-macromolecular compound containing phosphorus having no phosphorus-to-carbon bond
- C10M137/04—Phosphate esters
- C10M137/10—Thio derivatives
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M141/00—Lubricating compositions characterised by the additive being a mixture of two or more compounds covered by more than one of the main groups C10M125/00 - C10M139/00, each of these compounds being essential
- C10M141/10—Lubricating compositions characterised by the additive being a mixture of two or more compounds covered by more than one of the main groups C10M125/00 - C10M139/00, each of these compounds being essential at least one of them being an organic phosphorus-containing compound
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M159/00—Lubricating compositions characterised by the additive being of unknown or incompletely defined constitution
- C10M159/12—Reaction products
- C10M159/18—Complexes with metals
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M159/00—Lubricating compositions characterised by the additive being of unknown or incompletely defined constitution
- C10M159/12—Reaction products
- C10M159/20—Reaction mixtures having an excess of neutralising base, e.g. so-called overbasic or highly basic products
- C10M159/22—Reaction mixtures having an excess of neutralising base, e.g. so-called overbasic or highly basic products containing phenol radicals
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M159/00—Lubricating compositions characterised by the additive being of unknown or incompletely defined constitution
- C10M159/12—Reaction products
- C10M159/20—Reaction mixtures having an excess of neutralising base, e.g. so-called overbasic or highly basic products
- C10M159/24—Reaction mixtures having an excess of neutralising base, e.g. so-called overbasic or highly basic products containing sulfonic radicals
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2207/00—Organic non-macromolecular hydrocarbon compounds containing hydrogen, carbon and oxygen as ingredients in lubricant compositions
- C10M2207/02—Hydroxy compounds
- C10M2207/023—Hydroxy compounds having hydroxy groups bound to carbon atoms of six-membered aromatic rings
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2207/00—Organic non-macromolecular hydrocarbon compounds containing hydrogen, carbon and oxygen as ingredients in lubricant compositions
- C10M2207/02—Hydroxy compounds
- C10M2207/023—Hydroxy compounds having hydroxy groups bound to carbon atoms of six-membered aromatic rings
- C10M2207/026—Hydroxy compounds having hydroxy groups bound to carbon atoms of six-membered aromatic rings with tertiary alkyl groups
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2207/00—Organic non-macromolecular hydrocarbon compounds containing hydrogen, carbon and oxygen as ingredients in lubricant compositions
- C10M2207/02—Hydroxy compounds
- C10M2207/023—Hydroxy compounds having hydroxy groups bound to carbon atoms of six-membered aromatic rings
- C10M2207/027—Neutral salts thereof
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2207/00—Organic non-macromolecular hydrocarbon compounds containing hydrogen, carbon and oxygen as ingredients in lubricant compositions
- C10M2207/02—Hydroxy compounds
- C10M2207/023—Hydroxy compounds having hydroxy groups bound to carbon atoms of six-membered aromatic rings
- C10M2207/028—Overbased salts thereof
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2207/00—Organic non-macromolecular hydrocarbon compounds containing hydrogen, carbon and oxygen as ingredients in lubricant compositions
- C10M2207/09—Metal enolates, i.e. keto-enol metal complexes
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2207/00—Organic non-macromolecular hydrocarbon compounds containing hydrogen, carbon and oxygen as ingredients in lubricant compositions
- C10M2207/10—Carboxylix acids; Neutral salts thereof
- C10M2207/14—Carboxylix acids; Neutral salts thereof having carboxyl groups bound to carbon atoms of six-membered aromatic rings
- C10M2207/144—Carboxylix acids; Neutral salts thereof having carboxyl groups bound to carbon atoms of six-membered aromatic rings containing hydroxy groups
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2207/00—Organic non-macromolecular hydrocarbon compounds containing hydrogen, carbon and oxygen as ingredients in lubricant compositions
- C10M2207/10—Carboxylix acids; Neutral salts thereof
- C10M2207/14—Carboxylix acids; Neutral salts thereof having carboxyl groups bound to carbon atoms of six-membered aromatic rings
- C10M2207/146—Carboxylix acids; Neutral salts thereof having carboxyl groups bound to carbon atoms of six-membered aromatic rings having carboxyl groups bound to carbon atoms of six-membeered aromatic rings having a hydrocarbon substituent of thirty or more carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2207/00—Organic non-macromolecular hydrocarbon compounds containing hydrogen, carbon and oxygen as ingredients in lubricant compositions
- C10M2207/26—Overbased carboxylic acid salts
- C10M2207/262—Overbased carboxylic acid salts derived from hydroxy substituted aromatic acids, e.g. salicylates
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2207/00—Organic non-macromolecular hydrocarbon compounds containing hydrogen, carbon and oxygen as ingredients in lubricant compositions
- C10M2207/28—Esters
- C10M2207/287—Partial esters
- C10M2207/289—Partial esters containing free hydroxy groups
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant compositions
- C10M2215/02—Amines, e.g. polyalkylene polyamines; Quaternary amines
- C10M2215/04—Amines, e.g. polyalkylene polyamines; Quaternary amines having amino groups bound to acyclic or cycloaliphatic carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant compositions
- C10M2215/02—Amines, e.g. polyalkylene polyamines; Quaternary amines
- C10M2215/04—Amines, e.g. polyalkylene polyamines; Quaternary amines having amino groups bound to acyclic or cycloaliphatic carbon atoms
- C10M2215/042—Amines, e.g. polyalkylene polyamines; Quaternary amines having amino groups bound to acyclic or cycloaliphatic carbon atoms containing hydroxy groups; Alkoxylated derivatives thereof
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant compositions
- C10M2215/02—Amines, e.g. polyalkylene polyamines; Quaternary amines
- C10M2215/06—Amines, e.g. polyalkylene polyamines; Quaternary amines having amino groups bound to carbon atoms of six-membered aromatic rings
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant compositions
- C10M2215/02—Amines, e.g. polyalkylene polyamines; Quaternary amines
- C10M2215/06—Amines, e.g. polyalkylene polyamines; Quaternary amines having amino groups bound to carbon atoms of six-membered aromatic rings
- C10M2215/064—Di- and triaryl amines
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant compositions
- C10M2215/02—Amines, e.g. polyalkylene polyamines; Quaternary amines
- C10M2215/06—Amines, e.g. polyalkylene polyamines; Quaternary amines having amino groups bound to carbon atoms of six-membered aromatic rings
- C10M2215/064—Di- and triaryl amines
- C10M2215/065—Phenyl-Naphthyl amines
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant compositions
- C10M2215/02—Amines, e.g. polyalkylene polyamines; Quaternary amines
- C10M2215/06—Amines, e.g. polyalkylene polyamines; Quaternary amines having amino groups bound to carbon atoms of six-membered aromatic rings
- C10M2215/066—Arylene diamines
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant compositions
- C10M2215/02—Amines, e.g. polyalkylene polyamines; Quaternary amines
- C10M2215/06—Amines, e.g. polyalkylene polyamines; Quaternary amines having amino groups bound to carbon atoms of six-membered aromatic rings
- C10M2215/067—Polyaryl amine alkanes
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant compositions
- C10M2215/02—Amines, e.g. polyalkylene polyamines; Quaternary amines
- C10M2215/06—Amines, e.g. polyalkylene polyamines; Quaternary amines having amino groups bound to carbon atoms of six-membered aromatic rings
- C10M2215/068—Amines, e.g. polyalkylene polyamines; Quaternary amines having amino groups bound to carbon atoms of six-membered aromatic rings having amino groups bound to polycyclic aromatic ring systems, i.e. systems with three or more condensed rings
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant compositions
- C10M2215/086—Imides
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant compositions
- C10M2215/26—Amines
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2215/00—Organic non-macromolecular compounds containing nitrogen as ingredients in lubricant compositions
- C10M2215/28—Amides; Imides
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2217/00—Organic macromolecular compounds containing nitrogen as ingredients in lubricant compositions
- C10M2217/04—Macromolecular compounds from nitrogen-containing monomers obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds
- C10M2217/046—Polyamines, i.e. macromoleculars obtained by condensation of more than eleven amine monomers
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2217/00—Organic macromolecular compounds containing nitrogen as ingredients in lubricant compositions
- C10M2217/06—Macromolecular compounds obtained by functionalisation op polymers with a nitrogen containing compound
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2219/00—Organic non-macromolecular compounds containing sulfur, selenium or tellurium as ingredients in lubricant compositions
- C10M2219/04—Organic non-macromolecular compounds containing sulfur, selenium or tellurium as ingredients in lubricant compositions containing sulfur-to-oxygen bonds, i.e. sulfones, sulfoxides
- C10M2219/044—Sulfonic acids, Derivatives thereof, e.g. neutral salts
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2219/00—Organic non-macromolecular compounds containing sulfur, selenium or tellurium as ingredients in lubricant compositions
- C10M2219/04—Organic non-macromolecular compounds containing sulfur, selenium or tellurium as ingredients in lubricant compositions containing sulfur-to-oxygen bonds, i.e. sulfones, sulfoxides
- C10M2219/046—Overbasedsulfonic acid salts
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2219/00—Organic non-macromolecular compounds containing sulfur, selenium or tellurium as ingredients in lubricant compositions
- C10M2219/08—Thiols; Sulfides; Polysulfides; Mercaptals
- C10M2219/082—Thiols; Sulfides; Polysulfides; Mercaptals containing sulfur atoms bound to acyclic or cycloaliphatic carbon atoms
- C10M2219/087—Thiols; Sulfides; Polysulfides; Mercaptals containing sulfur atoms bound to acyclic or cycloaliphatic carbon atoms containing hydroxy groups; Derivatives thereof, e.g. sulfurised phenols
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2219/00—Organic non-macromolecular compounds containing sulfur, selenium or tellurium as ingredients in lubricant compositions
- C10M2219/08—Thiols; Sulfides; Polysulfides; Mercaptals
- C10M2219/082—Thiols; Sulfides; Polysulfides; Mercaptals containing sulfur atoms bound to acyclic or cycloaliphatic carbon atoms
- C10M2219/087—Thiols; Sulfides; Polysulfides; Mercaptals containing sulfur atoms bound to acyclic or cycloaliphatic carbon atoms containing hydroxy groups; Derivatives thereof, e.g. sulfurised phenols
- C10M2219/088—Neutral salts
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2219/00—Organic non-macromolecular compounds containing sulfur, selenium or tellurium as ingredients in lubricant compositions
- C10M2219/08—Thiols; Sulfides; Polysulfides; Mercaptals
- C10M2219/082—Thiols; Sulfides; Polysulfides; Mercaptals containing sulfur atoms bound to acyclic or cycloaliphatic carbon atoms
- C10M2219/087—Thiols; Sulfides; Polysulfides; Mercaptals containing sulfur atoms bound to acyclic or cycloaliphatic carbon atoms containing hydroxy groups; Derivatives thereof, e.g. sulfurised phenols
- C10M2219/089—Overbased salts
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2223/00—Organic non-macromolecular compounds containing phosphorus as ingredients in lubricant compositions
- C10M2223/02—Organic non-macromolecular compounds containing phosphorus as ingredients in lubricant compositions having no phosphorus-to-carbon bonds
- C10M2223/04—Phosphate esters
- C10M2223/045—Metal containing thio derivatives
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2227/00—Organic non-macromolecular compounds containing atoms of elements not provided for in groups C10M2203/00, C10M2207/00, C10M2211/00, C10M2215/00, C10M2219/00 or C10M2223/00 as ingredients in lubricant compositions
- C10M2227/06—Organic compounds derived from inorganic acids or metal salts
- C10M2227/061—Esters derived from boron
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10M—LUBRICATING COMPOSITIONS; USE OF CHEMICAL SUBSTANCES EITHER ALONE OR AS LUBRICATING INGREDIENTS IN A LUBRICATING COMPOSITION
- C10M2227/00—Organic non-macromolecular compounds containing atoms of elements not provided for in groups C10M2203/00, C10M2207/00, C10M2211/00, C10M2215/00, C10M2219/00 or C10M2223/00 as ingredients in lubricant compositions
- C10M2227/09—Complexes with metals
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2010/00—Metal present as such or in compounds
- C10N2010/02—Groups 1 or 11
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2010/00—Metal present as such or in compounds
- C10N2010/04—Groups 2 or 12
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2010/00—Metal present as such or in compounds
- C10N2010/06—Groups 3 or 13
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2010/00—Metal present as such or in compounds
- C10N2010/08—Groups 4 or 14
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2010/00—Metal present as such or in compounds
- C10N2010/10—Groups 5 or 15
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2010/00—Metal present as such or in compounds
- C10N2010/12—Groups 6 or 16
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2010/00—Metal present as such or in compounds
- C10N2010/14—Group 7
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2010/00—Metal present as such or in compounds
- C10N2010/16—Groups 8, 9, or 10
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2040/00—Specified use or application for which the lubricating composition is intended
- C10N2040/25—Internal-combustion engines
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2040/00—Specified use or application for which the lubricating composition is intended
- C10N2040/25—Internal-combustion engines
- C10N2040/251—Alcohol fueled engines
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2040/00—Specified use or application for which the lubricating composition is intended
- C10N2040/25—Internal-combustion engines
- C10N2040/252—Diesel engines
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2040/00—Specified use or application for which the lubricating composition is intended
- C10N2040/25—Internal-combustion engines
- C10N2040/252—Diesel engines
- C10N2040/253—Small diesel engines
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2040/00—Specified use or application for which the lubricating composition is intended
- C10N2040/25—Internal-combustion engines
- C10N2040/255—Gasoline engines
-
- C—CHEMISTRY; METALLURGY
- C10—PETROLEUM, GAS OR COKE INDUSTRIES; TECHNICAL GASES CONTAINING CARBON MONOXIDE; FUELS; LUBRICANTS; PEAT
- C10N—INDEXING SCHEME ASSOCIATED WITH SUBCLASS C10M RELATING TO LUBRICATING COMPOSITIONS
- C10N2040/00—Specified use or application for which the lubricating composition is intended
- C10N2040/25—Internal-combustion engines
- C10N2040/255—Gasoline engines
- C10N2040/28—Rotary engines
Definitions
- This invention relates to lubricating oils having improved wear properties, particularly oils for crankcase lubrication of gasoline and/or diesel engines.
- Lubricating oils for use in gasoline and diesel crankcases require regular improvement in performance to meet the demands of spark-ignited and diesel engines which are continually being uprated.
- the lubricating oils used in the engines need to have improved performance in respect of wear control and a reduction in the formation of undersirable deposits, such as varnish, sludge, carbonaceous and resinous materials which can form in the engines, adhering to the engine components and reducing the efficiency of the engine.
- ashless dispersants which are typically oil-soluble additives comprising long chain hydrocarbon polymers substituted with mono- or dicarboxylic acid producing moieties, preferably dicarboxylic acid or anhydride moieties.
- polymers such as ethylene-propylene copolymers and terpolymers containing non- conjugated dienes, have been disclosed as suitable polymers for the preparation of ashless nitrogen and ester dispersants.
- US-A-4234435 discloses dispersants prepared from polyalkenes of Mn between 1300 and 5000.
- the polyalkene can comprise homopolymers or interpolymers of C2 to C16 terminal olefins, of which ethylene- propylene copolymers are given as examples, with specific reference to a copolymer of 80% ethylene and 20% propylene.
- a new class of ashless dispersants comprising functionalised and/or derivatised olefin polymers, particularly ethylene/alpha-olefin polymers, has been produced using metallocene catalyst systems.
- Such dispersants are described in US-A-5128056, 5151204, 5200103, 5225092, 5266223, 5334775; WO-A-94/ 19436, 94/13709; and EP-A-440506, 513157 and 51321 1. These dispersants have improved viscometric properties as expressed in a ratio of CCS viscosity to kV 100°C.
- Useful dispersants of this type have a hydrocarbon polymer moiety with a Mn of 700 to 5000, preferably 1600 to 3500.
- ashless dispersants are effective for reducing engine deposits and the concentration of ashless dispersant in the lubricating oils can be increased to meet the increase in intervals between oil changes required by motor manufacturers.
- concentration of ashless dispersant in the lubricating oils can be increased to meet the increase in intervals between oil changes required by motor manufacturers.
- higher engine wear can occur, particularly when the engine is operating under low temperature conditions. This increase in wear is believed to arise from complex interactions between the ashless dispersants and other additives in the lubricating oil, particularly the metal salts of dihydrocarbyl phosphorodithioic acids, usually present in combination with the ashless dispersants.
- EP-A-379566 discloses a lubricating oil composition for internal combustion engines which comprises
- (B-l) at least one substituted succinic acylating agent with (B-2) at least one amine compound characterized by the presence within its structure of at least one HN ⁇ group
- said substituted succinic acylating agents consist of substituent groups and succinic groups wherein the substituent groups are derived from polyalkene, said polyalkene being characterized by a M n value of 1300 to about 5000 and a M w / M n value of about 1.5 to about 4.5, said acylating agents being characterized by the presence within their structure of an average of at least 1.3 succinic groups for each equivalent weight of substituent groups, and (C) from about 0.05 to about 5% by weight of a mixture of metal salts of dihydrocarbyl phosphorodithioic acids wherein in at least one of the dihydrocarbyl phosphorodithioic acids, one of the hydrocarbyl groups (C-l) is an isopropyl or secondary butyl group, the other hydrocarbyl
- the specification describes the preparation of a variety of dihydrocarbyl phosphorodithioic metal salts but contains no exemplification of compositions containing the additives by which to judge their effectiveness.
- the problem of the increased wear brought about using combinations of ashless dispersants and the phosphorodithioic metal salts is not disclosed.
- WO 89/06237 discloses the preparation of basic metal dihydrocarbylphosphorodithioates and phosphoromonothioates by employing a catalytic amount of alkali or alkaline earth metal hydroxide or mixtures thereof. However, it does not address the problem of wear under low temperature conditions.
- US-A-4529526 discloses a lubricating oil composition
- a lubricating oil composition comprising: a) oil; b) sulfurised oxymetal organophosphorodithioate of a specific formula and/or sulfurised oxymetal dithiocarbamate of a specific formula; c) at least one ZDDP of a specific formula; d) calcium benzenesulfonate and/or calcium petroleumsulfonate; and e) alkenylsuccinic acid imide and/or boron derivatives thereof.
- Such a composition is described as providing superior reduction in mechanical friction loss of four-cycle engines.
- the dispersants specifically disclosed in the specification have polymers with number average molecular weight of less than 1000.
- a lubricating oil composition such as for crankcase lubrication of gasoline or diesel engines, obtained by mixing:
- a second aspect of the present invention provides a lubricating oil concentrate comprising 3 to 60 weight percent of component (B) and 1 to 20 weight percent of component (C) in a base oil of lubricating viscosity, wherein components (B) and (C) are defined as in the first aspect.
- a third aspect of the present invention provides the use in a lubricating oil composition of a dihydrocarbyl phosphorodithioic acid metal salt according to component (C) defined in the first aspect for improving the wear protection of spark-ignited and diesel engines under low temperature conditions.
- a fourth aspect of the present invention provides the use in spark-ignited or diesel engines of a lubricating oil composition as defined in the first aspect for improving the wear protection of said spark-ignited or diesel engines.
- the oil of lubricating viscosity may be selected from natural (vegetable, animal or mineral) and synthetic lubricating oils and mixtures thereof. It may range in viscosity from light distillate mineral oils to heavy lubricating oils such as gas engine oil, mineral lubricating oil, motor vehicle oil, and heavy duty diesel oil. Generally, the viscosity of the oil ranges from 2 centistokes to 30 centistokes, especially 5 centistokes to 20 centistokes, at 100°C as measured by ASTM D445.
- Natural oils include animal oils and vegetable oils (e.g., castor, lard oil) liquid petroleum oils and hydrorefined, solvent-treated or acid-treated mineral lubricating oils of the paraffinic, naphthenic and mixed paraffinic-naphthenic types. Oils of lubricating viscosity derived from coal or shale are also useful base oils.
- animal oils and vegetable oils e.g., castor, lard oil
- mineral lubricating oils of the paraffinic, naphthenic and mixed paraffinic-naphthenic types.
- Oils of lubricating viscosity derived from coal or shale are also useful base oils.
- Synthetic lubricating oils include hydrocarbon oils and halo-substituted hydrocarbon oils such as polymerized and interpolymerized olefins (e.g., polybutylenes, polypropylenes, propylene-isobutylene copolymers, chlorinated polybutylenes, poly(l-hexenes), poly(l- octenes), poly(l-decenes)); alkylbenzenes (e.g., dodecylbenzenes, tetradecylbenzenes, dinonylbenzenes, di(2-ethylhexyl)benzenes); polyphenyls (e.g., biphenyls, terphenyls, alkylated polyphenols); and alkylated diphenyl ethers and alkylated diphenyl sulfides and the derivatives; analogs and homologs thereof. Also suitable are poly alpha-olefin synthetic
- Alkylene oxide polymers and interpolymers and derivatives thereof where the terminal hydroxyl groups have been modified by esterification, etherification, etc. constitute another class of known synthetic lubricating oils. These are exemplified by polyoxyalkylene polymers prepared by polymerization of ethylene oxide or propylene oxide, the alkyl and aryl ethers of these polyoxyalkylene polymers (e.g., methylpolyisopropylene glycol ether having an average molecular weight of 1000, diphenyl ether of poly-ethylene glycol having a molecular weight of 500-1000, diethyl ether of polypropylene glycol having a molecular weight of 1000-1500); and mono- and polycarboxylic esters thereof, for example, the acetic acid esters, mixed C3-C8 fatty acid esters and C13 Oxo acid diester of tetraethylene glycol.
- polyoxyalkylene polymers prepared by polymerization of ethylene oxide or propylene oxide
- Another suitable class of synthetic lubricating oils comprises the esters of dicarboxylic acids (e.g., phthalic acid, succinic acid, alkyl succinic acids and alkenyl succinic acids, maleic acid, azelaic acid, subericacid, sebasic acid, fumaric acid, adipic acid, linoleic acid dimer, malonic acid, alkylmalonic acids, alkenyl malonic acids) with a variety of alcohols (e.g., butyl alcohol, hexyl alcohol, dodecyl alcohol, 2-ethylhexyl alcohol, ethylene glycol, diethylene glycol monoether, propylene glycol).
- dicarboxylic acids e.g., phthalic acid, succinic acid, alkyl succinic acids and alkenyl succinic acids, maleic acid, azelaic acid, subericacid, sebasic acid, fumaric acid, adipic acid, linole
- esters include dibutyl adipate, di(2-ethylhexyl) sebacate, di-n-hexyl fumarate, dioctyl sebacate, di- isooctyl azelate, di-isodecyl azelate, dioctyl phthalate, didecyl phthalate, dieicosyl sebacate, the 2-ethylhexyl diester of linoleic acid dimer, and the complex ester formed by reacting one mole of sebacic acid with two moles of tetraethylene glycol and two moles of 2-ethylhexanoic acid.
- Esters useful as synthetic oils also include those made from C5 to C12 monocarboxylic acids and polyols and polyol ethers such as neopentyl glycol, trimethylolpropane, pentaerythritol, dipentaerythritol and tripentaerythritol.
- Silicon-based oils such as the polyalkyl-, polyaryl-, polyalkoxy-, or polyaryloxysiloxane oils and silicate oils comprise another useful class of synthetic lubricants; they include tetraethyl silicate, tetraisopropyl silicate, tetra-(2-ethylhexyl)silicate, tetra-(4-methyl-2- ethylhexyl)silicate, tetra-(p-tert-butyl-phenyl)silicate, hexa-(4-methyl-2- pentoxy)disiloxane, poly(methyl)siloxanes and poly(n ethylphenyl)siloxanes.
- Other synthetic lubricating oils include liquid esters of phosphorus-containing acids (e.g., tricresyl phosphate, trioctyl phosphate, diethyl ester of decylphosphonic acid) and polymeric tetrahydrofurans.
- Unrefined, refined and rerefined oils can be used in the lubricants of the present invention.
- Unrefined oils are those obtained directly from a natural or synthetic source without further purification treatment.
- a shale oil obtained directly from retorting operations a petroleum oil obtained directly from distillation or ester oil obtained directly from an esterification process and used without further treatment would be an unrefined oil.
- Refined oils are similar to the unrefined oils except they have been further treated in one or more purification steps to improve one or more properties. Many such purification techniques, such as distillation, solvent extraction, acid or base extraction, filtration and percolation are known to those skilled in the art. Rerefined oils are obtained by processes similar to those used to obtain refined oils applied to refined oils which have been already used in service. Such rerefined oils are also known as reclaimed or reprocessed oils and often are additionally processed by techniques for removal of spent additives and oil breakdown products.
- At least 0.0006 mole, of ashless dispersant, per 100 g of the total composition may be used in the present invention, more preferably at least 0.00061, especially at least 0.00062 mole per 100 g of the total composition.
- the oil compositions of the present invention use at most 0.0014 mole, of ashless dispersant, per 100 g of the total composition, preferably at most 0.0013, such as at most 0.0012 mole per 100 g of the total composition.
- the ashless dispersant may be employed in an amount of at least 1.92, more preferably at least 2.56, most preferably at least 2.60, advantageously at least 2.65 % by weight based on the weight of the total composition; and at most 6.00, preferably at most 5.50, such as at most 5.10 % by weight based on the weight of the total composition.
- the high molecular weight ashless dispersants of the composition of the invention include the range of ashless dispersants known as effective for adding to lubricant oils for the purpose of reducing the formation of deposits in gasoline or diesel engines. A wide variety of such compounds is available, as now described in more detail.
- the ashless dispersant comprises an oil soluble polymeric hydrocarbon backbone having functional groups that are capable of associating with particles to be dispersed.
- the dispersants comprise amine, alcohol, amide, or ester polar moieties attached to the polymer backbone often via a bridging group.
- the ashless dispersant may be, for example, selected from oil soluble salts, esters, amino-esters, amides, imides, and oxazolines of long chain hydrocarbon substituted mono and dicarboxylic acids or their anhydrides; thiocarboxylate derivatives of long chain hydrocarbons; long chain aliphatic hydrocarbons having a polyamine attached directly thereto; and Mannich condensation products formed by condensing a long chain substituted phenol with formaldehyde and polyalkylene polyamine.
- a class of ashless dispersants comprising ethylene alpha-olefin copolymers and alpha- olefin homo- and copolymers prepared using new metallocene catalyst chemistry, which may have a high degree (e.g. >30%) of terminal vinylidene unsaturation is described in US-A-5128056, 5151204, 5200103, 5225092, 5266223, 5334775; WO-A-94/19436, 94/13709; and EP-A-440506, 513157, 513211. These dispersants are described as having superior viscometric properties as expressed in a ratio of CCS viscosity to kV 100°C.
- alpha-olefin is used herein to denote an olefin of the formula
- R' is preferably a C1-C18 alkyl group.
- the requirement for terminal vinylidene unsaturation refers to the presence in the polymer of the following structure:
- Poly is the polymer chain and R is typically a C1-C18 alkyl group, typically methyl or ethyl.
- R is typically a C1-C18 alkyl group, typically methyl or ethyl.
- the polymers will have at least 50%, and most preferably at least 60%, of the polymer chains with terminal vinylidene unsaturation.
- ethylene/1-butene copolymers typically have vinyl groups terminating no more than about 10 percent of the chains, and internal mono-unsaturation in the balance of the chains. The nature of the unsaturation may be determined by FTIR spectroscopic analysis, titration or C-13 NMR.
- the oil-soluble polymeric hydrocarbon backbone may be a homopolymer (e.g., polypropylene) or a copolymer of two or more of such olefins: for example, copolymers of ethylene and an alpha-olefin such as an alpha-olefin containing up to 8, preferably up to 6, such as up to 4 carbon atoms (e.g. propylene, butylene, hexene or octene), or copolymers of two different alpha-olefins.
- a homopolymer e.g., polypropylene
- a copolymer of two or more of such olefins for example, copolymers of ethylene and an alpha-olefin such as an alpha-olefin containing up to 8, preferably up to 6, such as up to 4 carbon atoms (e.g. propylene, butylene, hexene or octene), or copoly
- copolymers include those in which a minor molar amount of the copolymer monomers, e.g., 1 to 10 mole %, is an ⁇ , ⁇ -diene, such as a C3 to C22 non-conjugated diolefin (e.g., a copolymer of isobutylene and butadiene, or a copolymer of ethylene, propylene and 1 ,4-hexadiene or 5-ethylidene-2-norbornene).
- Atactic propylene oligomers typically having a Mn of from 1000 to 4000 may also be used, as described in EP-A-490454, as well as heteropolymers such as polyepoxides.
- olefin polymers are polybutenes and specifically poly-n-butenes, such as may be prepared by polymerization of a C4 refinery stream.
- Other preferred classes of olefin polymers are EAO copolymers that preferably contain 1 to 50 mole % ethylene, and more preferably 5 to 48 mole % ethylene. Such polymers may contain more than one alpha-olefin and may contain one or more C3 to C22 diolefins. Also usable are mixtures of EAO's of varying ethylene content. Different polymer types, e.g., EAO, may also be mixed or blended, as well as polymers differing in Mn ; components derived from these also may be mixed or blended.
- the olefin polymers and copolymers used in the dispersant employed in the invention preferably have an Mn of from 1000 to 4000, more preferably at least 1100, advantageously at least 1200, for example 1300 to 4000, especially 1600 to 4000, such as from 2000 to 4000.
- Polymer molecular weight, specifically Mn can be determined by various known techniques. One convenient method is gel permeation chromatography (GPC), which additionally provides molecular weight distribution information (see W. W. Yau, J. J. Kirkland and D. D. Bly, "Modern Size Exclusion Liquid Chromatography", John Wiley and Sons, New York, 1979).
- GPC gel permeation chromatography
- Another useful method, particularly for lower molecular weight polymers is vapor pressure osmometry (see, e.g., ASTM D3592).
- the degree of polymerisation Dp of a polymer is:
- the degree of polymerisation for the polymer backbones used in the invention is at least 45, typically from 50 to 165, more preferably 55 to 140.
- Particularly preferred copolymers are ethylene butene copolymers.
- the olefin polymers and copolymers may be prepared by various catalytic polymerization processes using metallocene catalysts which are, for example, bulky ligand transition metal compounds of the formula:
- L is a bulky ligand
- A is a leaving group
- M is a transition metal
- m and n are such that the total ligand valency corresponds to the transition metal valency.
- the catalyst is four co-ordinate such that the compound is ionizable to a i valency state.
- the ligands L and A may be bridged to each other, and if two ligands A and/or L are present, they may be bridged.
- the metallocene compound may be a full sandwich compound having two or more ligands L which may be cyclopentadienyl ligands or cyclopentadienyl derived ligands, or they may be half sandwich compounds having one such ligand L.
- the ligand may be mono- or polynuclear or any other ligand capable of ⁇ -5 bonding to the transition metal.
- One or more of the ligands may ⁇ -bond to the transition metal atom, which may be a Group 4, 5 or 6 transition metal and/or a lanthanide or actinide transition metal, with zirconium, titanium and hafnium being particularly preferred.
- the transition metal atom which may be a Group 4, 5 or 6 transition metal and/or a lanthanide or actinide transition metal, with zirconium, titanium and hafnium being particularly preferred.
- the ligands may be substituted or unsubstituted, and mono-, di-, tri, tetra- and penta- substitution of the cyclopentadienyl ring is possible.
- the substituent(s) may act as one or more bridges between the ligands and/or leaving groups and/or transition metal.
- Such bridges typically comprise one or more of a carbon, germanium, silicon, phosphorus or nitrogen atom-containing radical, and preferably the bridge places a one-atom link between the entities being bridged, although that atom may and often does carry other substituents.
- the metallocene may also contain a further displaceable ligand, preferably displaced by a cocatalyst - a leaving group - that is usually selected from a wide variety of hydrocarbyl groups and halogens.
- the oil-soluble polymeric hydrocarbon backbone may be functionalized to inco ⁇ orate a functional group into the backbone of the polymer, or as one or more groups pendant from the polymer backbone.
- the functional group typically will be polar and contain one or more hetero atoms such as P, O, S, N, halogen, or boron. It can be attached to a saturated hydrocarbon part of the oil-soluble polymeric hydrocarbon backbone via substitution reactions or to an olefinic portion via addition or cycloaddition reactions. Alternatively, the functional group can be inco ⁇ orated into the polymer in conjunction with oxidation or cleavage of the polymer chain end (e.g., as in ozonolysis).
- Useful functionalization reactions include: halogenation of the polymer at an olefinic bond and subsequent reaction of the halogenated polymer with an ethylenically unsaturated functional compound (e.g., maleation where the polymer is reacted with maleic acid or anhydride); reaction of the polymer with an unsaturated functional compound by the "ene" reaction absent halogenation; reaction of the polymer with at least one phenol group (this permits derivatization in a Mannich base-type condensation); reaction of the polymer at a point of unsaturation with carbon monoxide using a Koch-type reaction to introduce a carbonyl group in an iso or neo position; reaction of the polymer with the functionalizing compound by free radical addition using a free radical catalyst; reaction with a thiocarboxylic acid derivative; and reaction of the polymer by air oxidation methods, epoxidation, chloroamination, or ozonolysis.
- an ethylenically unsaturated functional compound e
- the functionalized oil-soluble polymeric hydrocarbon backbone is then further derivatized with a nucleophilic reactant such as an amine, amino-alcohol, alcohol, metal compound or mixture thereof to form a corresponding derivative.
- a nucleophilic reactant such as an amine, amino-alcohol, alcohol, metal compound or mixture thereof.
- Useful amine compounds for derivatizing functionalized polymers comprise at least one amine and can comprise one or more additional amine or other reactive or polar groups. These amines may be hydrocarbyl amines or may be predominantly hydrocarbyl amines in which the hydrocarbyl group includes other groups, e.g., hydroxy groups, alkoxy groups, amide groups, nitriles, imidazoline groups, and the like.
- Particularly useful amine compounds include mono- and polyamines, e.g.
- polyalkylene and polyoxyalkylene polyamines of about 2 to 60, conveniently 2 to 40 (e.g., 3 to 20), total carbon atoms and about 1 to 12, conveniently 3 to 12, and preferably 3 to 9 nitrogen atoms in the molecule.
- Mixtures of amine compounds may advantageously be used such as those prepared by reaction of alkylene dihalide with ammonia.
- Preferred amines are aliphatic saturated amines, including, e.g., 1,2- diaminoethane; 1,3-diaminopropane; 1,4-diaminobutane; 1 ,6-diaminohexane; polyethylene amines such as diethylene triamine; triethylene tetramine; tetraethylene pentamine; and polypropyleneamines such as 1 ,2-propylene diamine; and di-(l,2-propylene)triamine.
- 1,2- diaminoethane 1,3-diaminopropane
- 1,4-diaminobutane 1,4-diaminobutane
- 1 ,6-diaminohexane polyethylene amines such as diethylene triamine; triethylene tetramine; tetraethylene pentamine
- polypropyleneamines such as 1 ,2-propylene diamine; and di-(l,2-propylene
- amine compounds include: alicyclic diamines such as 1 ,4-di(aminomethyl) cyclohexane, and heterocyclic nitrogen compounds such as imidazolines.
- a particularly useful class of amines are the polyamido and related amido-amines as disclosed in US 4,857,217; 4,956,107; 4,963,275; and 5,229,022.
- THAM tris(hydroxymethyl)amino methane
- Dendrimers, star-like amines, and comb-structure amines may also be used.
- the functionalized oil-soluble polymeric hydrocarbon backbones also may be derivatized with hydroxy compounds such as monohydric and polyhydric alcohols or with aromatic compounds such as phenols and naphthols.
- Polyhydric alcohols are preferred, e.g., alkylene glycols in which the alkylene radical contains from 2 to 8 carbon atoms.
- Other useful polyhydric alcohols include glycerol, mono-oleate of glycerol, monostearate of glycerol, monomethyl ether of glycerol, pentaerythritol, dipentaerythritol, and mixtures thereof.
- An ester dispersant may also be derived from unsaturated alcohols such as allyl alcohol, cinnamyl alcohol, propargyl alcohol, l-cyclohexane-3-ol, and oleyl alcohol.
- unsaturated alcohols such as allyl alcohol, cinnamyl alcohol, propargyl alcohol, l-cyclohexane-3-ol, and oleyl alcohol.
- Still other classes of the alcohols capable of yielding ashless dispersants comprise the ether- alcohols and including, for example, the oxy-alkylene, oxy-arylene. They are exemplified by ether-alcohols having up to 150 oxy-alkylene radicals in which the alkylene radical contains from 1 to 8 carbon atoms.
- the ester dispersants may be di-esters of succinic acids or acidic esters, i.e., partially esterified succinic acids, as well as partially esterified polyhydric alcohols or phenols, i.e., esters having free alcohols or phenolic hydroxyl radicals.
- An ester dispersant may be prepared by one of several known methods as illustrated, for example, in US 3,381,022.
- a preferred group of ashless dispersants includes those substituted with succinic anhydride groups and reacted with polyethylene amines (e.g., tetraethylene pentamine), aminoalcohols such as trismethylolaminomethane and optionally additional reactants such as alcohols and reactive metals e.g., pentaerythritol, and combinations thereof). Also useful are dispersants wherein a polyamine is attached directly to the backbone by the methods shown in US 3,275,554 and 3,565,804 where a halogen group on a halogenated hydrocarbon is displaced with various alkylene polyamines.
- polyethylene amines e.g., tetraethylene pentamine
- aminoalcohols such as trismethylolaminomethane
- additional reactants such as alcohols and reactive metals e.g., pentaerythritol, and combinations thereof.
- dispersants wherein a polyamine is attached directly to the backbone by the methods
- Mannich base condensation products are prepared by condensing about one mole of an alkyl-substituted mono- or polyhydroxy benzene with about 1 to 2.5 moles of carbonyl compounds (e.g., formaldehyde and paraformaldehyde) and about 0.5 to 2 moles polyalkylene polyamine as disclosed, for example, in US 3,442,808.
- carbonyl compounds e.g., formaldehyde and paraformaldehyde
- Such Mannich condensation products may include a polymer product of a metallocene cataylsed polymerisation as a substituent on the benzene group or may be reacted with a compound containing such a polymer substituted on a succinic anhydride, in a manner similar to that shown in US 3,442,808.
- Examples of functionalized and/or derivatized olefin polymers based on polymers synthesized using metallocene catalyst systems are described in publications identified above.
- the dispersant can be further post-treated by a variety of conventional post treatments such as boration, as generally taught in US 3,087,936 and 3,254,025.
- This is readily accomplished by treating an acyl nitrogen-containing dispersant with a boron compound selected from the group consisting of boron oxide, boron halides, boron acids and esters of boron acids, in an amount to provide from about 0.1 atomic proportion of boron for each mole of the acylated nitrogen composition to about 20 atomic proportions of boron for each atomic proportion of nitrogen of the acylated nitrogen composition.
- the dispersants contain from about 0.05 to 2.0 wt. %, e.g. 0.05 to 0.7 wt.
- boron based on the total weight of the borated acyl nitrogen compound.
- the boron which appears be in the product as dehydrated boric acid polymers (primarily (HBO2)3), is believed to attach to the dispersant imides and diimides as amine salts e.g., the metaborate salt of the diimide.
- Boration is readily carried out by adding from about 0.05 to 4, e.g., 1 to 3 wt. % (based on the weight of acyl nitrogen compound) of a boron compound, preferably boric acid, usually as a slurry, to the acyl nitrogen compound and heating with stirring at from 135° to 190° C, e.g., 140°-170° C, for from 1 to 5 hours followed by nitrogen stripping.
- a boron compound preferably boric acid, usually as a slurry
- the boron treatment can be carried out by adding boric acid to a hot reaction mixture of the dicarboxylic acid material and amine while removing water.
- the dispersants which are most effective at associating with and suspending foreign particles in the engine oil are also observed to reduce the effectiveness of the antiwear agents to the greatest extent, and exert their deleterious effect at lower concentrations of ashless dispersant than less effective ashless dispersants.
- the invention is particularly useful for providing improved wear to those lubricating compositions containing ashless dispersants which are most effective in reducing engine deposits.
- compositions of the invention are those containing ashless dispersants based on poly(isobutylene) polymers having a number average molecular weight of from 1300 to 2500, preferably 1600 to 2500, more preferably 2000 to 2500, substituted with succinic anhydride groups which have been further functionalised.
- the dispersant contains at least 1.0, and desirably at least 1.3 succinic groups per polymer group.
- a preferred functionalising class of compounds contains at least one NH ⁇ group.
- functionalisation is effected using from 0.5 equivalents to 2 moles of amine compound per equivalent of succinic anhydride substituted polymer.
- ashless dispersants are the functionalised and derivatised olefin polymers based on ethylene alpha-olefin polymers previously described, produced using metallocene catalyst systems. These, preferably, have number average molecular weights of from 1600 to 3500, more preferably 2000 to 3500, especially 2500 to 3500.
- ashless dispersants comprising a polymeric hydrocarbon backbone having number average molecular weight less than 1000 may also be used in combination with ashless dispersants of the invention.
- component (C) may be used in the present invention in a range of from 0.00055 to 0.0019 mole per 100 g of the total composition, more preferably from 0.0006 to 0.00185, especially from 0.0007 to 0.0017 mole per 100 g of the total composition.
- the metal salt may be used in a range of from 0.330 to 1.320, preferably from 0.363 to 1.255, more preferably from 0.396 to 1.221, most preferably from 0.462 to 1.122 % by weight based on the weight of the total composition.
- hydrocarbyl denotes a radical having a carbon atoms directly attached to the remainder of the molecule and having predominatly hydrocarbon character within the context of this invention.
- radicals include the following:
- Hydrocarbon radicals that is, aliphatic, e.g. alkyl or alkenyl, alicyclic (e.g. cycloalkyl or cycloalkenyl), aromatic, aliphatic- and alicyclic-substituted aromatic, aromatic-substituted aliphatic and alicylic radicals, as well as cyclic radicals wherein the ring is completed through another portion of the molecule (that is, any two indicated substituents may together form an alicylic radical).
- aliphatic e.g. alkyl or alkenyl
- alicyclic e.g. cycloalkyl or cycloalkenyl
- aromatic aliphatic- and alicyclic-substituted aromatic
- aromatic-substituted aliphatic and alicylic radicals as well as cyclic radicals wherein the ring is completed through another portion of the molecule (that is, any two indicated substituents may together form an alicylic radical).
- Substituted hydrocarbon radicals that is, radicals containing non-hydrocarbon substituents which, in the context of this invention, do not alter the predominantly hydrocarbon character of the radical.
- substituents examples are halo (particularly chloro and fluoro), alkoxy, mercapto, nitro, nitroso, sulfoxy, and other groups.
- Hetero radicals that is, radicals which, while predominantly hydrocarbon in character within the context of this invention, contain atoms other than carbon in a chain or ring otherwise composed of carbon atoms. Suitable hetero atoms will be apparent to those skilled in the art and include, for example, nitrogen, oxygen, and sulfur.
- the hydrocarbyl groups are alkyl groups and may be linear or branched; preferred are branched alkyl groups.
- the metal salt component may be a mixture of salts provided that the dialkyl content of the mixture of salts conforms to the limits set out above.
- dialkyl salt 100 mole % of the dialkyl groups are secondary alkyl groups containing 6 carbon atoms, i.e. if a single metal salt is used both alkyl groups of the compound, denoted by Rl and R2, are secondary C6 alkyl groups.
- the minimum molar percentage of secondary C6 alkyl groups in component (C) is 70 mole %, i.e. at least 70 mole % of the total of Rl and R2 are secondary C6 alkyl groups, preferably at least 80, more preferably at least 90 mole %.
- the remainder of the alkyl groups in the compound are selected from primary groups, secondary groups having less than 6 carbon atoms and secondary groups having more than 6 carbon atoms, provided that there are present no more than 15, preferably no more than 10, especially no more than 5 mole % of either primary groups or secondary groups containing less than 6 carbon atoms.
- component (C) may be a mixture of a salt of a dihydrocarbyl phosphorodithoic acid in which 100 mole % of the alkyl groups are secondary C6 groups, in admixture with other dialkyl phosphorodithoic acid salts provided that the total alkyl group concentrations of the mixture fall within the prescribed limits.
- a pruned secondary C6 alkyl group is 4-methyl-2-pentyl.
- Other suitable secondary C6 groups include the isomers of methyl-pentyl, dimethyl butyl and ethyl butyl, for example 2,3-dimethyl-2-butyl, 3,3-dimethyl-2-butyl and 3-methyl-2-pentyl.
- the primary groups may include isobutyl, n-butyl, n-amyl, n-hexyl, n-heptyl, 2-ethyl- hexyl, iso-octyl, nonyl and higher alkyl groups.
- the secondary groups containing less than 6 carbon atoms include sec-butyl. Secondary groups containing more than 6 carbon atoms preferably contain no more than 10 carbon atoms.
- the preferred metal salt of the dihydrocarbyl phosphordithoic acid is zinc, for example those represented by the formula Zn[SP(S)(OR')(OR 2 )] 2 , where R 1 and R 2 are defined as above.
- DDPA dihydrocarbyl dithiophosphoric acid
- P2S5 dihydrocarbyl dithiophosphoric acid
- multiple dithiophosphoric acids can be prepared where the hydrocarbyl groups on one are entirely secondary in character and the hydrocarbyl groups on the others are entirely primary in character.
- zinc salt any basic or neutral zinc compound could be used but the oxides, hydroxides and carbonates are most generally employed. Commercial additives frequently contain an excess of zinc due to use of an excess of the basic zinc compound in the neutralization reaction.
- Neutral, or normal, and basic metal salts of dihydrocarbyl phosphordithioic acids can be employed within the present invention.
- basic it is meant those salts that have a higher ratio of equivalents of total metal to equivalents of the dihydrocarbyl phosphordithioic acid than that of the corresponding "neutral” or "normal” salt.
- Neutral, or normal, metal salts have one equivalent of metal per one equivalent of the dihydrocarbyl phosphordithioic acid.
- Components (B) and (C) must be "oil-soluble” or “oil-dispersible” in the oil of lubricating viscosity, but these do not mean that they are soluble, dissolvable, miscible or capable of being suspended in the oil in all proportions. They do mean, however, that (B) and (C) are, for instance, soluble or stably dispersible in the oil to an extent sufficient to exert their intended effect in the environment in which the lubricating oil composition is employed. Moreover, the additional inco ⁇ oration of other additives such as those described hereinafter may affect the oil-solubility or dispersibility of one or both of (B) and (C).
- compositions of the invention include compositions in which interaction between any of the additive components has occurred, as well as compositions in which no interaction has occurred between the components mixed in the lubricating oil.
- Components (B) and (C) may be mixed with the oil of lubricating viscosity (component (A)) in any convenient way.
- each of (B) and (C) can be added directly to the oil by dispersing or dissolving in the oil at the desired level of concentration at ambient or elevated temperature. They may be added individually or together to the oil.
- Oil concentrate Example of suitable oleaginous carriers are oils of lubricating viscosity, such as those described in detail hereinbefore, and aliphatic, naphthenic and aromatic hydrocarbons.
- concentrates there may be present from 1 to 90% by weight of the total weight of concentrate a mixture of components (B) and (C), preferably from 10 to 90% by weight, more preferably from 20 to 85% by weight.
- the ashless dispersant (B) is present in an amount of from 3 to 60% by weight of the concentrate, more preferably from 20 to 60 % by weight, most preferably from 30 to 60 % by weight of the concentrate.
- the dihydrocarbyl phosphorodithioic acid metal salt (C) is preferably present in an amount of from 1 to 20% by weight of the concentrate, more preferably from 3 to 19 % by weight, most preferably from 5 to 17 % by weight of the concentrate.
- One or more additional additives, such as described hereinafter, may also be present in such concentrates.
- additives may be inco ⁇ orated in the compositions of the invention to enable them to meet particular requirements.
- additives which may be included in the lubricating oil compositions are detergents and metal rust inhibitors, viscosity index improvers, corrosion inhibitors, other oxidation inhibitors, friction modifiers, other dispersants, anti-foaming agents, anti-wear agents, pour point depressants, and rust inhibitors. Some are discussed in further detail below.
- Detergents and metal rust inhibitors include, for example, the oil-soluble sulphonates, phenates, sulphurized phenates, thiophosphonates, salicylates, and naphthenates and other oil-soluble carboxylates of a metal, particularly the alkali or alkaline earth metals or magnesium, for example, sodium, lithium, calcium, barium and magnesium.
- the most commonly used metals are calcium and magnesium, mixtures of calcium and magnesium, and mixtures of calcium and/or magnesium with sodium.
- the detergents may be overbased: overbased detergents function both as detergents and acid neutralizers, thereby reducing wear and corrosion and extending engine life.
- suitable viscosity modifiers are polyisobutylene, copolymers of ethylene and propylene, polymethacrylates, methacrylate copolymers, copolymers of an unsaturated dicarboxylic acid and a vinyl compound, inte ⁇ olymers of styrene and acrylic esters, and partially hydrogenated copolymers of styrene/ isoprene, styrene/butadiene, and isoprene/butadiene, as well as the partially hydrogenated homopolymers of butadiene and isoprene.
- supplementary antioxidants include, for example, aromatic amines, for example alkylated phenylamines and phenyl ⁇ -naphthylamine; hindered phenols; alkaline earth metal salts of sulphurized alkyl-phenols having preferably C5 to C12 alkyl side chains, e.g., calcium nonylphenyl sulphide; barium octylphenyl sulphide; hindered phenols; phosphosulphurized or sulphurized hydrocarbons; and oil-soluble copper compounds.
- aromatic amines for example alkylated phenylamines and phenyl ⁇ -naphthylamine
- hindered phenols alkaline earth metal salts of sulphurized alkyl-phenols having preferably C5 to C12 alkyl side chains, e.g., calcium nonylphenyl sulphide; barium octylphenyl sulph
- Friction modifiers and fuel economy agents which are compatible with the other ingredients of the final oil may also be included.
- examples of such materials are molybdenum compounds and glyceryl monoesters of higher fatty acids, for example, glyceryl mono-oleate; esters of long chain polycarboxylic acids with diols, for example, the butane diol ester of a dimerized unsaturated fatty acid; oxazoline compounds; and alkoxylated alkyl-substituted mono-amines, diamines and alkyl ether amines, for example, ethoxylated tallow amine and ethoxylated tallow ether amine.
- a viscosity index improver dispersant functions both as a viscosity index improver and as a dispersant.
- examples of viscosity index improver dispersants include reaction products of amines, for example polyamines, with a hydrocarbyl-substituted mono- or dicarboxylic acid in which the hydrocarbyl substituent comprises a chain of sufficient length to impart viscosity index improving properties to the compounds.
- the viscosity index improver dispersant may be, for example, a polymer of a C4 to C24 unsaturated ester of vinyl alcohol or a C3 to CIO unsaturated mono-carboxylic acid or a C4 to CIO dicarboxylic acid with an unsaturated nitrogen-containing monomer having 4 to 20 carbon atoms; a polymer of a C2 to C20 olefin with an unsaturated C3 to CIO mono- or dicarboxylic acid neutralised with an amine, hydroxyamine or an alcohol; or a polymer of ethylene with a C3 to C20 olefin further reacted either by grafting a C4 to C20 unsaturated nitrogen - containing monomer thereon or by grafting an unsaturated acid onto the polymer backbone and then reacting carboxylic acid groups of the grafted acid with an amine, hydroxy amine or alcohol.
- dispersants and viscosity index improver dispersants may be found in European Patent Specification No. 24146 B.
- Pour point depressants otherwise known as lube oil flow improvers, lower the minimum temperature at which the fluid will flow or can be poured.
- Such additives are well known. Typical of those additives which improve the low temperature fluidity of the fluid are C8 to C18 dialkyl fumarate/vinyl acetate copolymers, and polymethacrylates.
- Foam control can be provided by an antifoamant of the polysiloxane type, for example, silicone oil or polydimethyl siloxane.
- additives can provide a multiplicity of effects; thus for example, a single additive may act as a dispersant-oxidation inhibitor. This approach is well known and need not be further elaborated herein.
- each additive is typically blended into the base oil in an amount which enables the additive to provide its desired function.
- additive concentrates comprising the additives (concentrates sometimes being referred to as additive packages) whereby several additives can be added simultaneously to the oil to form the lubricating oil composition.
- the final composition may employ typically about 10 wt % of the additive-package, the remainder being oil of lubricating viscosity.
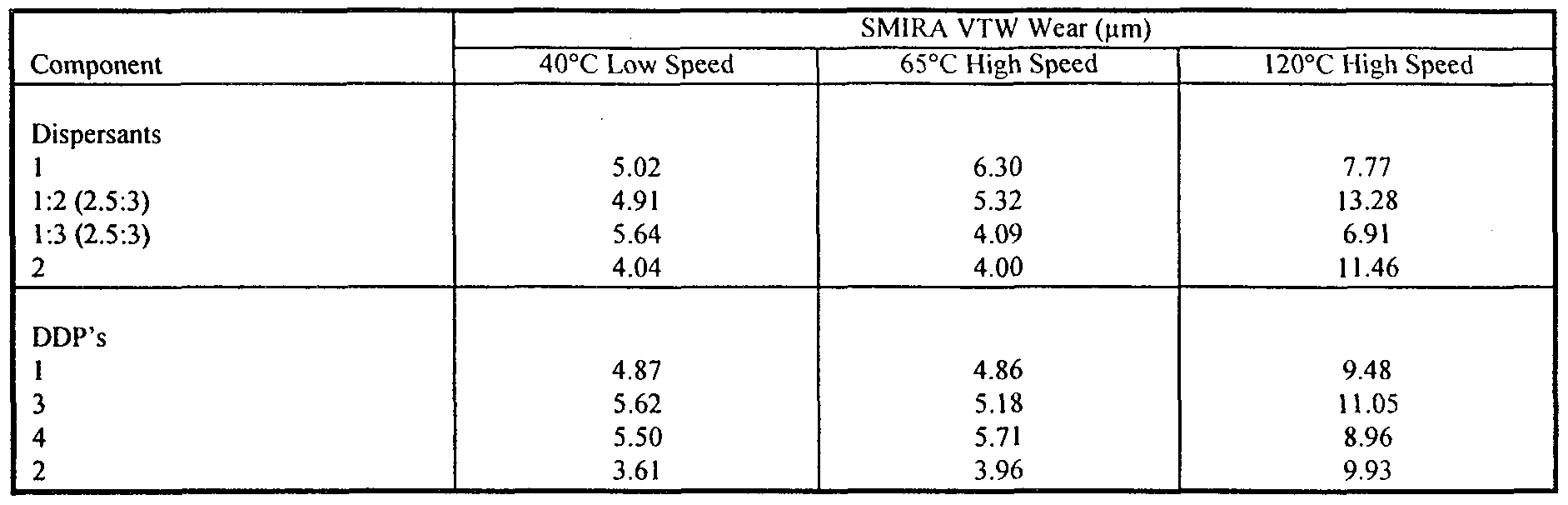
- the wear properties of the compositions of the invention are evaluated in the following Example using the SMIRA Valve Train Wear Rig test, the results being expressed as wear scar, diameter, measured in ⁇ m.
- the SMIRA test is described in a publication by CH. Bovington and A. Hubbard, entitled “Friction and Wear in the Internal Combustion Engine", I. Mech. E., London, 1989. This describes a motored single cam and follower arrangement in which load speed and temperature can be varied. The impact on wear is assessed by the difference in the size ⁇ f the HV 30 mark before and after the test.
- the Example shows the results of a statistically designed experiment evaluating the effect of variations of different zinc salts of various dialkyl phosphorodithioic acids in combination with various ashless dispersants, overbased and neutral metal detergents and antioxidants. These are typically present as components in lubricating oil compositions. It is necessary to evaluate the results on the basis of the statistical significance of the results because there are complex interactions between the various components of the compositions which are difficult to separate and inte ⁇ ret unless such a mathematical approach is adopted.
- Example A series of compositions containing the components shown in Table 1 were made up in a base lubricating oil according to the following procedure.
- the weights indicated in Table 1 are those of the additive, that is the active ingredient of the additive plus the diluent or oil.
- a batch of base oil containing a viscosity modifier was made up by blending 6.2 parts by weight of an ethylene/propylene copolymer in a mixture of solvent refined 100 neutral and 150 neutral oil of American manufacture (87.6 parts by weight 100N to 6.2 parts by weight 150N).
- the mixture of oil and viscosity modifier was stirred at 70°C for 1 hr.
- compositions listed in Table 1 were made up using aliquot portions of this base oil mixture.
- the blending of the listed ingredients was effected by adding the dispersant to a portion of the oil mixture and maintaining the mixture at 80°C for 2 hours. After cooling to 60°C the overbased and neutral detergents, zinc salt and antioxidant were added and the blend was maintained at 60°C for 1 hr.
- Dispersant 3 As Dispersant 2 but having a number average molecular weight of 950 (PIB chain).
- DDP 2 As DDP 1, but containing 100 mole % of 4-methyl-2- pentyl groups. DDP 2 is an example of a metal salt of the present invention.
- DDP 3 As DDP 1, but containing 77 mole % of isobutyl groups and 23 mole % of iso-amyl groups.
- DDP 4 As DDP 1 , but containing 100 mole % of iso-octyl groups.
- Antioxidant 1 A copper salt of polyisobutenyl succinimide.
- Antioxidant 2 Nonyl phenol sulpide.
- Antioxidant 3 A hindered phenol.
- Antioxidant 4 A diphenylamine antioxidant.
- Table 2 The results are shown in Table 2. Analysis of these results is shown in Tables 3 and 4. Table 3 shows the contribution the dispersant and the ZDDP confers to the measured wear property.
- the zinc salts provide the largest contribution (30.1%) under the low speed and low temperature condition; it is almost three times the unexplained variation in the experiment and is statistically significant at the 93% confidence interval. At high temperature, the zinc salts are less important, the dispersant having a larger impact.
- the zinc salt (2) conforming to the present invention has the best performance (the lowest wear, 3.61 ⁇ m) compared to the zinc salts (1), (2) and (3).
- the dispersant combination (1 :3) of high and low number average molecular weight for the polymer gave poor wear control indicating the preference for ashless dispersants comprising a polymer backbone with a selected number average molecular weight range.
Abstract
Description
Claims
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AU70428/98A AU7042898A (en) | 1997-03-27 | 1998-03-23 | Lubricating compositions |
| EP98917105A EP1115815A1 (en) | 1997-03-27 | 1998-03-23 | Lubricating compositions |
| CA002288812A CA2288812A1 (en) | 1997-03-27 | 1998-03-23 | Lubricating compositions |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| GB9706468.7 | 1997-03-27 | ||
| GBGB9706468.7A GB9706468D0 (en) | 1997-03-27 | 1997-03-27 | Intermediate chain length ZDDP with high Mn dispersants give improved wear |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO1998044079A1 true WO1998044079A1 (en) | 1998-10-08 |
Family
ID=10810026
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP1998/001899 WO1998044079A1 (en) | 1997-03-27 | 1998-03-23 | Lubricating compositions |
Country Status (6)
| Country | Link |
|---|---|
| EP (1) | EP1115815A1 (en) |
| AR (1) | AR012201A1 (en) |
| AU (1) | AU7042898A (en) |
| CA (1) | CA2288812A1 (en) |
| GB (1) | GB9706468D0 (en) |
| WO (1) | WO1998044079A1 (en) |
Cited By (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1999060080A1 (en) * | 1998-05-15 | 1999-11-25 | Infineum Usa L.P. | Lubricant compositions for and their use in internal combustion engines |
| EP1027412A1 (en) * | 1997-11-12 | 2000-08-16 | Exxon Chemical Patents Inc. | Wear control with dispersants employing poly alpha-olefin polymers |
| EP1136544A1 (en) * | 2000-03-20 | 2001-09-26 | Infineum International Limited | Crankcase lubricating oil composition |
| JP2001303087A (en) * | 2000-03-20 | 2001-10-31 | Infineum Internatl Ltd | Lubricating oil composition |
| EP1172430A2 (en) * | 2000-06-29 | 2002-01-16 | Bridgestone Corporation | Lubricant composition for steel filament and rubber-steel filament composite body |
| WO2002088284A1 (en) * | 2001-05-01 | 2002-11-07 | Infineum International Limited | Combustion improving additive for small engine lubricating oils |
| WO2015021135A1 (en) * | 2013-08-09 | 2015-02-12 | The Lubrizol Corporation | Reduced engine deposits from dispersant treated with copper |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1365311A (en) * | 1972-03-10 | 1974-08-29 | Orobis Ltd | Lubricants |
| EP0113045A1 (en) * | 1982-11-30 | 1984-07-11 | Honda Motor Co., Ltd. | Lubricating oil composition |
| EP0242405A1 (en) * | 1984-10-05 | 1987-10-28 | Asahi Denka Kogyo Kabushiki Kaisha | Sulfidoxymolybdenum dialkylphosphorodithioate |
| US4704215A (en) * | 1985-09-03 | 1987-11-03 | Idemitsu Kosan Company Limited | Lubricant composition for transmission of power |
| EP0304011A1 (en) * | 1987-08-19 | 1989-02-22 | Kyodo Oil Technical Research Center Co., Ltd. | Lubricating oil composition for internal combustion engine |
| JPH08302378A (en) * | 1995-04-28 | 1996-11-19 | Nippon Oil Co Ltd | Engine oil composition |
| US5744430A (en) * | 1995-04-28 | 1998-04-28 | Nippon Oil Co., Ltd. | Engine oil composition |
-
1997
- 1997-03-27 GB GBGB9706468.7A patent/GB9706468D0/en active Pending
-
1998
- 1998-03-23 EP EP98917105A patent/EP1115815A1/en not_active Withdrawn
- 1998-03-23 CA CA002288812A patent/CA2288812A1/en not_active Abandoned
- 1998-03-23 WO PCT/EP1998/001899 patent/WO1998044079A1/en not_active Application Discontinuation
- 1998-03-23 AU AU70428/98A patent/AU7042898A/en not_active Abandoned
- 1998-03-27 AR ARP980101426A patent/AR012201A1/en unknown
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1365311A (en) * | 1972-03-10 | 1974-08-29 | Orobis Ltd | Lubricants |
| EP0113045A1 (en) * | 1982-11-30 | 1984-07-11 | Honda Motor Co., Ltd. | Lubricating oil composition |
| EP0242405A1 (en) * | 1984-10-05 | 1987-10-28 | Asahi Denka Kogyo Kabushiki Kaisha | Sulfidoxymolybdenum dialkylphosphorodithioate |
| US4704215A (en) * | 1985-09-03 | 1987-11-03 | Idemitsu Kosan Company Limited | Lubricant composition for transmission of power |
| EP0304011A1 (en) * | 1987-08-19 | 1989-02-22 | Kyodo Oil Technical Research Center Co., Ltd. | Lubricating oil composition for internal combustion engine |
| JPH08302378A (en) * | 1995-04-28 | 1996-11-19 | Nippon Oil Co Ltd | Engine oil composition |
| US5744430A (en) * | 1995-04-28 | 1998-04-28 | Nippon Oil Co., Ltd. | Engine oil composition |
Non-Patent Citations (1)
| Title |
|---|
| PATENT ABSTRACTS OF JAPAN vol. 097, no. 003 31 March 1997 (1997-03-31) * |
Cited By (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1027412A4 (en) * | 1997-11-12 | 2002-01-02 | Exxonmobil Chem Patents Inc | Wear control with dispersants employing poly alpha-olefin polymers |
| EP1027412A1 (en) * | 1997-11-12 | 2000-08-16 | Exxon Chemical Patents Inc. | Wear control with dispersants employing poly alpha-olefin polymers |
| WO1999060080A1 (en) * | 1998-05-15 | 1999-11-25 | Infineum Usa L.P. | Lubricant compositions for and their use in internal combustion engines |
| SG100648A1 (en) * | 2000-03-20 | 2003-12-26 | Infineum Int Ltd | Lubricating oil compositions |
| JP2001303087A (en) * | 2000-03-20 | 2001-10-31 | Infineum Internatl Ltd | Lubricating oil composition |
| US6423670B2 (en) | 2000-03-20 | 2002-07-23 | Infineum International Ltd. | Lubricating oil compositions |
| EP1136544A1 (en) * | 2000-03-20 | 2001-09-26 | Infineum International Limited | Crankcase lubricating oil composition |
| JP2012162750A (en) * | 2000-03-20 | 2012-08-30 | Infineum Internatl Ltd | Lubricating oil composition |
| EP1172430A2 (en) * | 2000-06-29 | 2002-01-16 | Bridgestone Corporation | Lubricant composition for steel filament and rubber-steel filament composite body |
| EP1172430A3 (en) * | 2000-06-29 | 2002-04-03 | Bridgestone Corporation | Lubricant composition for steel filament and rubber-steel filament composite body |
| WO2002088284A1 (en) * | 2001-05-01 | 2002-11-07 | Infineum International Limited | Combustion improving additive for small engine lubricating oils |
| WO2015021135A1 (en) * | 2013-08-09 | 2015-02-12 | The Lubrizol Corporation | Reduced engine deposits from dispersant treated with copper |
| CN105637073A (en) * | 2013-08-09 | 2016-06-01 | 路博润公司 | Reduced engine deposits from dispersant treated with copper |
| US20160298052A1 (en) * | 2013-08-09 | 2016-10-13 | The Lubrizol Corporation | Reduced engine deposits from dispersant treated with copper |
Also Published As
| Publication number | Publication date |
|---|---|
| GB9706468D0 (en) | 1997-05-14 |
| EP1115815A1 (en) | 2001-07-18 |
| CA2288812A1 (en) | 1998-10-08 |
| AR012201A1 (en) | 2000-09-27 |
| AU7042898A (en) | 1998-10-22 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US6060437A (en) | Lubricating oil compositions | |
| US6140279A (en) | Concentrates with high molecular weight dispersants and their preparation | |
| US5726134A (en) | Multigrade lubricating compositions | |
| EP1027412B1 (en) | Wear control with dispersants employing etylene- alpha-olefin polymers | |
| EP0757711B2 (en) | Crankcase lubricant for modern heavy duty diesel and gasoline fueled engines | |
| US6310010B1 (en) | High molecular weight dispersant compositions and their preparation | |
| US5789355A (en) | Low volatility lubricating compositions | |
| EP1115815A1 (en) | Lubricating compositions | |
| EP0765370B1 (en) | Multigrade lubricating compositions containing no viscosity modifier | |
| EP1070111B1 (en) | Process for the preparation of oleginous concentrates | |
| CA2327829C (en) | Concentrates with high molecular weight dispersants and their preparation | |
| EP0777713B1 (en) | Improved lubricating oil compositions | |
| US5652202A (en) | Lubricating oil compositions | |
| EP0765372B1 (en) | Low volatility luricating compositions | |
| EP0765371A1 (en) | Shear stable lubricating compositions |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 09091568 Country of ref document: US |
|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AU BR CA JP KR MX SG US |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): AT BE CH DE DK ES FI FR GB GR IE IT LU MC NL PT SE |
|
| DFPE | Request for preliminary examination filed prior to expiration of 19th month from priority date (pct application filed before 20040101) | ||
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 1998917105 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref document number: 2288812 Country of ref document: CA Ref country code: CA Ref document number: 2288812 Kind code of ref document: A Format of ref document f/p: F |
|
| NENP | Non-entry into the national phase |
Ref country code: JP Ref document number: 1998541164 Format of ref document f/p: F |
|
| WWP | Wipo information: published in national office |
Ref document number: 1998917105 Country of ref document: EP |
|
| WWW | Wipo information: withdrawn in national office |
Ref document number: 1998917105 Country of ref document: EP |