US20110054081A1 - Formulation and its use - Google Patents
Formulation and its use Download PDFInfo
- Publication number
- US20110054081A1 US20110054081A1 US12/552,338 US55233809A US2011054081A1 US 20110054081 A1 US20110054081 A1 US 20110054081A1 US 55233809 A US55233809 A US 55233809A US 2011054081 A1 US2011054081 A1 US 2011054081A1
- Authority
- US
- United States
- Prior art keywords
- formulation according
- component
- acid
- acrylate
- radical
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 0 [1*]OCC(C)CC Chemical compound [1*]OCC(C)CC 0.000 description 25
Classifications
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B26/00—Compositions of mortars, concrete or artificial stone, containing only organic binders, e.g. polymer or resin concrete
- C04B26/02—Macromolecular compounds
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B24/00—Use of organic materials as active ingredients for mortars, concrete or artificial stone, e.g. plasticisers
- C04B24/24—Macromolecular compounds
- C04B24/243—Phosphorus-containing polymers
- C04B24/246—Phosphorus-containing polymers containing polyether side chains
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B28/00—Compositions of mortars, concrete or artificial stone, containing inorganic binders or the reaction product of an inorganic and an organic binder, e.g. polycarboxylate cements
- C04B28/14—Compositions of mortars, concrete or artificial stone, containing inorganic binders or the reaction product of an inorganic and an organic binder, e.g. polycarboxylate cements containing calcium sulfate cements
- C04B28/145—Calcium sulfate hemi-hydrate with a specific crystal form
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08J—WORKING-UP; GENERAL PROCESSES OF COMPOUNDING; AFTER-TREATMENT NOT COVERED BY SUBCLASSES C08B, C08C, C08F, C08G or C08H
- C08J9/00—Working-up of macromolecular substances to porous or cellular articles or materials; After-treatment thereof
- C08J9/0066—Use of inorganic compounding ingredients
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B2111/00—Mortars, concrete or artificial stone or mixtures to prepare them, characterised by specific function, property or use
- C04B2111/00474—Uses not provided for elsewhere in C04B2111/00
- C04B2111/00612—Uses not provided for elsewhere in C04B2111/00 as one or more layers of a layered structure
- C04B2111/0062—Gypsum-paper board like materials
Definitions
- the subject of the present invention is a formulation for the dispersion of hydraulic binder and especially gypsum containing compositions.
- Usual dispersants are static in their chemical structure over time in hydraulic systems. Their performance is controlled by monomer molar ratio that is fixed within a polymer molecule. A water reducing effect or dispersing effect is observed upon dispersant adsorption onto the hydraulic particle surface. As dispersant demand increases over time due to abrasion and hydration product formation, which creates more surface area, these conventional dispersants are unable to respond and workability is lost.
- Various types of organic compounds have been used to advantageously alter certain properties of wet hydraulic binder compositions.
- One class of components which can collectively be called “superplasticizers” fluidify or plasticize wet binder compositions to obtain a more fluid mixture.
- a controlled fluidity is desired, such that the aggregate used in mortars and concretes does not segregate from the binder paste.
- superplasticizers may allow the cement composition to be prepared using a lower water: binder ratio in order to obtain a composition having a desired consistency which often leads to a hardened composition having a higher compressive strength development after setting.
- a good superplasticizer should not only fluidify the wet hydraulic binder composition to which it is added, but also maintain the level of fluidity over a desired period of time. This time should be long enough to keep the wet composition fluid, e. g. in a ready-mix truck while it is on its way to a job site. Another important aspect relates to the period for discharging the truck at the job site and the period needed for the cement composition for being worked in the desired final form. On the other side, the hydraulic mixture cannot remain fluid for a too long time, that means the set must not greatly be retarded, because this will slow down the work on the job and show negative influences on the characteristics of the final hardened products.
- superplasticizers are melamine sulfonate/formaldehyde condensation products, naphthalene sulfonate/formaldehyde condensation products and lignosulfonates, polysaccharides, hydroxycarboxylic acids and their salts and carbohydrates.
- fluidizing agents are multi-component products with copolymers based on oxyalkylenglykolalkenylethers and unsaturated dicarboxylic acid-derivatives as most important species.
- the European Patent EP 0 736 553 B1 discloses such copolymers comprising at least three sub-units and especially one unsaturated dicarboxylic acid derivative, one oxyalkylenglykolalkenylether and additionally one hydrophobic structural unit, such as ester units.
- the third structural unit can also be represented by polypropylenoxid- and polypropylenoxid-polyethylenoxid-derivatives, respectively.
- German published application DE 195 43 304 A1 discloses an additive for water containing mixtures for the construction field comprising a) a water-soluble sulfonic acid-, carboxylic- or sulfate group containing cellulose derivative, b) a sulfonic acid- and/or carboxylic acid containing vinyl-(co)-polymer and/or a condensation product based on aminoplast-builders or acryl containing compounds and formaldehyde.
- This additive shall show sufficient water retention ability and rheology-modifying properties. Therefore, this additive shall be suitable for construction chemical compositions containing cement, lime, gypsum, anhydrite and other hydraulic binder components.
- Copolymers based on unsaturated monocarboxylic or dicarboxylic acid derivatives, oxyalkylenglykolalkenylethers, vinylic polyalkylenglykol, polysiloxane or ester compounds used as additives for aqueous suspensions based on mineral or bituminous binders are described in U.S. Pat. No. 6,777,517 B1.
- the use of such additives results in a decrease in the water/binder ratio and leads to highly fluid building materials without segregation of individual constituents from the building material mixture.
- patents are useful as additives for aqueous suspensions of inorganic and organic solids and especially for suspensions that are based on mineral or bituminous binders such as cement, plaster of paris, lime, anhydrite or other building materials based on calcium sulfate.
- mineral or bituminous binders such as cement, plaster of paris, lime, anhydrite or other building materials based on calcium sulfate.
- copolymers of ethylenically unsaturated ethers that can be used as plasticizers for cement containing mixtures (EP 0 537 870 A1). These copolymers contain an ether co-monomer and as additional co-monomer an olefinic unsaturated mono-carboxylic acid or an ester or a salt thereof, or alternatively an olefinic unsaturated sulfuric acid. These copolymers show a very short ether side chain with 1 to 50 units. The short side chain causes a sufficient plasticizing effect of the copolymers in cement containing masses with a reduced slump loss of the construction chemicals mass itself.
- U.S. Pat. No. 6,139,623 B1 discloses an emulsion admixture for use in hydraulic cement compositions formed by emulsifying an antifoaming agent, a surfactant and a copolymer having a carbon-containing backbone to which are attached groups that function as cement-anchoring members by forming ionic bonds and oxyalkylene groups.
- This admixture comprising an ethylene oxide/propylene oxide (EO/PO) type comb polymer and an antifoaming agent allows a predictable air control in hydraulic cement compositions such as concrete.
- cement composition refers to pastes, mortars, grouts such as oil well cementing grouts, and concrete compositions comprising a hydraulic cement binder.
- Typical antifoaming agents are phosphate ester, borate ester and polyoxyalkylene copolymers with defoaming properties.
- the surface active component is said to stabilize the emulsion mixture and is chosen from the group consisting of an esterified fatty acid ester of a carbohydrate, a C 2 to C 20 alcohol having polyoxyalkylene groups or a mixture thereof.
- US 2006/0281886 discloses a co-polymer comprising two monomer components with a component a) being an olefinic unsaturated monocarboxylic acid co-monomer or an ester or a salt thereof or an olefinic unsaturated sulfonic acid co-monomer or a salt thereof, and with component b) preferably represented by an ether compound.
- component a) being an olefinic unsaturated monocarboxylic acid co-monomer or an ester or a salt thereof or an olefinic unsaturated sulfonic acid co-monomer or a salt thereof
- component b) preferably represented by an ether compound.
- These two monomeric co-polymer can be preferably used as a superplasticizer in a hydraulic binder containing composition.
- the co-polymer can be used in combination with a defoaming component that is also an additional structural unit of the co-polymer.
- the defoaming component can be chemically attached to the co-polymer or being present in free form in a blend.
- dispersing agents such as polycarboxylate ethers (PCE)
- PCE polycarboxylate ethers
- the workability and preferably the rheological behavior of the construction chemicals composition are improved.
- PCE based dispersants causes a distinct air entrainment to the binder component that worsens the physical properties of the composition.
- Another negative aspect is the foam formation during the preparation of the binder system.
- defoamer components are used as additional additive to the dispersing agent.
- defoamers show a low solubility in aqueous formulations and cause an insufficient stability.
- the defoaming properties of the formulation decrease over time due to the resulting phase separation of the defoamer and the dispersant.
- US 2008/017078 teaches a liquid admixture composition for a calcium sulfate based binder system and a method of use.
- the disclosed admixture comprises an aqueous composition of a copolymeric dispersing component, an antifoaming agent component, a surfactant component and water.
- the components may be a blend or physically or chemically attached and result in a stable liquid system that can be used as dispersing agent for calcium sulfate compound containing construction chemicals composition.
- the admixture composition disclosed in this document and especially its application as dispersing agent represent a further improvement of this state of the art because the admixture with its contained aqueous composition induces a uniform plasticizing effect all the time and an improvement of the physical properties due to reduction of both water and air content in the wet construction chemicals gypsum mass. Furthermore, the admixture shows an improved storage stability and homogeneity.
- Gypsum mixtures for foaming, solid and fast drying gypsum products and a method of making a gypsum slurry by using modifiers and dispersants are disclosed by US 2009/0101045, US 2006/0281837, US 2006/0280899, US 2006/0280898, US 2006/0278135, US 2006/0278134, US 2006/0278130, US 2006/0278127, US 2005/0250888, US 2005/0239924 and US 2006/0280970.
- the dispersants mentioned in these documents represent polycarboxylate dispersants, the dispersant having two repeating units with an olefinic unsaturated mono-carboxylic acid repeating unit and a vinyl or allyl-group bound to a polyether by an ether linkage as second repeating unit.
- a formulation for extending workability to a hydraulic binder and preferably a calcium sulfate containing mixture and water comprising introducing into the mixture a combination of dispersing components.
- the subject formulation achieves a better workability and fluidibility of hydraulic setting compositions and establishes a low water/hydraulic binder value.
- the present invention relates to a formulation containing
- structural unit (II) and structural unit (III) differing exclusively in that the OP(OH) 2 group of the structural unit (II) is replaced by H in structural unit (III), and structural unit (III) is not the same as structural unit (I), the formulation being suitable as admixture for a hydraulic binder and preferably a calcium sulfate binder system containing composition.
- hydroaulic binder means cement and preferably Portland cement represented by CEM I, CEM II, CEM III, CEM IV and CEM V, white cement, quick lime and aluminate cement.
- latent hydraulic binder means at least one representative selected from the group fly ash, blast furnace slag, metakaoline, microsilica, trass compounds, alumosilicates, tuff, phomulithe, diatomaceous earth and oil shell.
- calcium sulfate compound means calcium sulfate in its anhydrous and hydrate forms, such as gypsum, anhydrite, calcium sulfate dihydrate and calcium sulfate hemi-hydrate.
- gypsum is also known as calcium sulfate, whereby calcium sulfate can be used in its various anhydrous and hydrate forms with or without crystal water.
- Natural gypsum is represented by calcium sulfate dihydrate and the natural crystal water free form of calcium sulfate is represented by the term “anhydrite”.
- calcium sulfate is a typical by-product of technical processes characterized by the term “synthetic gypsum”.
- synthetic gypsum One example of such technical processes is the flue gas desulphurization.
- Synthetic gypsum may also be a by-product of phosphorous acid and hydrogen fluoride production methods for gaining hemi-hydrate forms (CaSO 4 1 ⁇ 2H 2 O).
- Gypsum (CaSO 4 .2H 2 O) may be calcinated by driving off the water of hydration. Products of the various calcinating procedures are alpha or beta hemi-hydrate. Beta calcium sulfate hemi-hydrate results from a rapid heating in open units by a rapid evaporation of water and by forming cavities. Alpha hemi-hydrate is produced by a de-watering of gypsum in closed autoclaves. The crystal form in this case is dense and therefore, this binder needs less amounts of water than beta hemi-hydrate.
- gypsum hemi-hydrate re-hydrates with water to dihydrate crystals.
- the hydration of gypsum needs some minutes to hours indicating a clearly shortened workability period in contrast to cements that hydrate in periods over hours or days.
- Calcium sulfate hemi-hydrate can produce at least two crystal forms, whereby ⁇ -calcined gypsum is usually de-watered (de-hydrated) in closed autoclaves.
- ⁇ -calcined gypsum may be selected due to its availability under economical aspects. However, these advantages may be reversed because ⁇ -calcined gypsum needs higher water amounts for workability and for making slurries of a given fluidity. Hardened or dried gypsum tends to a certain weakening based on the remained water in its crystal matrix. Therefore, products thereof show less strength than gypsum products that have been made with smaller amounts of water.
- the workability of gypsum, but also of other hydraulic binders, can be improved under hydraulic aspects by adding dispersants.
- the formulation according to this invention represents a suitable dispersant because of the dispersing properties of its component.
- Component a) of the formulation according to the invention has dispersing properties and is selected from the group consisting of a compound at least containing a branched comb polymer having polyether side chains, a naphthalene sulphonate-formaldehyde condensate (“BNS”), and a melamine sulphonate-formaldehyde condensate (“MSF”),
- Formulations which contain a branched comb polymer having polyether side chains as the component a) with dispersant action have been found extremely effective. It therefore can be seen as preferred embodiment that the component a) is a polycarboxylate ether a 1 ), a polycarboxylate ester a 2 ), an uncharged copolymer a 3 ) or a mixture thereof. In general and additionally to the dispersing properties of component a) polycarboxylate ester a 2 ) are preferred that show anti-foaming and surface active activities.
- Such polyether-containing copolymers which in the sense of the present invention are suitable as component a 1 ), have been previously described in WO 2006/133933 A2.
- These copolymers consist of two monomer components, the first monomer component being an olefinically unsaturated monocarboxylic acid comonomer or an ester or a salt thereof and/or an olefinically unsaturated sulphonic acid comonomer or a salt thereof, and the second monomer component a comonomer of the general formula (I)
- R 1 represents
- R 2 represents H or an aliphatic hydrocarbon residue with 1 to 5 C atoms
- R 3 unsubstituted or substituted aryl residue and preferably phenyl
- R 4 H or an aliphatic hydrocarbon residue with 1 to 20 C atoms, cycloaliphatic hydrocarbon residue with 5 to 8 C atoms, a substituted aryl residue with 6 to 14 C atoms or a member of the series
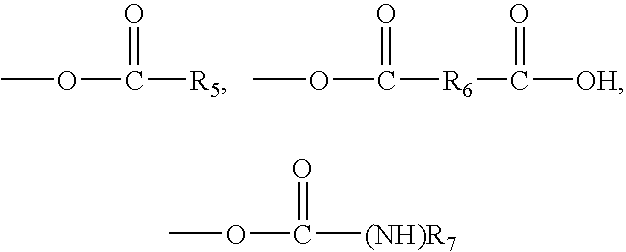
- R 5 and R 7 each represent an alkyl, aryl, aralkyl, or alkaryl residue and R 6 for an alkylidene, arylidene, aralkylidene or alkarylidene residue, and
- the present invention comprises a formulation wherein the copolymer a 1 ) contains the comonomer component 1) in proportions of 30 to 99 mol. % and the comonomer component 2) in proportions of 70 to 1 mol. %.
- a copolymer a 1 ) which contains the comonomer component 1) in proportions of 40 to 90 mol. % and the comonomer component 2) in proportions of 60 to 10 mol. % has been found particularly advantageous in this connection.
- the comonomer component 1) derives from the group acrylic acid, methacrylic acid, crotonic acid, isocrotonic acid, allylsulphonic acid, vinylsulphonic acid and suitable salts thereof and alkyl or hydroxyalkyl esters thereof.
- the copolymer a 1 can have additional structural groups in copolymerized form, which is also taken into account by the present invention.
- the additional structural groups may be styrenes, acrylamides and/or hydrophobic compounds, ester structural units, polypropylene oxide and polypropylene oxide/polyethylene oxide units being particularly preferred.
- the copolymer a 1 ) should contain the said additional structural groups in proportions up to 5 mol. %, preferably from 0.05 to 3.0 mol. % and in particular from 0.1 to 1.0 mol. %.
- this ester a 2 is a polymer which can be prepared by polymerization of a monomer mixture (I) containing, as the main component, a representative of the carboxylic acid monomer type.
- a monomer mixture (I) containing, as the main component, a representative of the carboxylic acid monomer type.
- An important aspect of component a 2 ) according to the present invention has to be seen in the anti-foaming and/or defoaming and/or surface active properties of such polycarboxylate ester types. This is why the formulation according to the present invention also comprises a combination of an antifoaming/surface active agent with dispersing properties as component a) and the polycondensate component b).
- the monomer mixture (I) contains an (alkoxy)polyalkylene glycol mono(meth)acrylate monomer (a) of the general formula (II)
- R 1 represents a hydrogen atom or a CH 3 group
- R 2 O represents one representative or a mixture of at least two oxyalkylene groups having 2 to 4 carbon atoms
- R 3 represents a hydrogen atom or an alkyl group having 1 to 5 carbon atoms
- m represents a number between 1 and 250 and represents the average number of moles of the oxyalkylene group added, additionally, as monomer (b), a (meth)acrylic acid of the general formula (III),
- R 4 represents a hydrogen atom or a CH 3 group and M 1 represents a hydrogen atom, a monovalent metal atom, a divalent metal atom, an ammonium group or an organic amine group, and optionally a monomer (c) which is copolymerized with the monomers (a) and (b).
- the monomer (a) can be present in an amount of from 5 to 98 wt. %, the monomer (b) in a proportion of from 2 to 95 wt. % and the monomer (c) in a proportion up to 50 wt. % in the monomer mixture (I), wherein the respective proportions of the monomers (a), (b) and (c) add up to 100 wt. %.
- the formulation according to the invention should contain at least one representative of the esters of an aliphatic alcohol with 1 to 20 carbon atoms with an unsaturated carboxylic acid.
- unsaturated carboxylic acid in particular maleic acid, fumaric acid, citraconic acid (meth)acrylic acid or monovalent metal salts, divalent metal salts, ammonium salts or organic amine salts thereof are especially suitable.
- Monoesters or diesters of unsaturated dicarboxylic acids such as maleic acid, fumaric acid or citraconic acid with aliphatic C 1 -C 20 alcohols, C 2 -C 4 glycols or with (alkoxy)polyalkylene glycols are preferred representatives of monomer (c) according to the present invention.
- the component a 2 can be a copolymer which is made up of at least one of the following monomers:
- components B) and C) are simultaneously represented in the copolymer a 2 ) of the claimed formulation.
- the ethylenically unsaturated monomer of the component A) can be at least one anhydride or imide and/or at least one maleic anhydride or maleimide.
- the ethylenically unsaturated monomer of the component A) can however also include an acrylate ester with an ester functionality which contains the hydrolysable residue. In this case, it should be regarded as preferred if the ester functionality is at least one hydroxypropyl or hydroxyethyl radical.
- the copolymer a 2 ) can however comprise more than one ethylenically unsaturated monomer with a hydrolysable radical.
- the ethylenically unsaturated monomer of the component A) as a residue has at least more than one representative of the ethylenically unsaturated monomers, at least one representative of a hydrolysable radical or a mixture of both.
- the hydrolysable radical should have at least one C 2 -C 20 alcohol functionality.
- the present invention also includes the possibility that the hydrolysable residue is at least one C 1 -C 20 alkyl ester, one C 1 -C 20 aminoalkyl ester, one C 2 -C 20 alcohol, one C 2 -C 20 amino alcohol or one amide.
- the present invention further comprises that at least one ethylenically unsaturated monomer of the component B) or C) has a C 2 -C 8 alkyl ether group.
- the ethylenically unsaturated monomer can have a vinyl, allyl or (methyl)allyl ether residue or else be derived from an unsaturated C 2 -C 8 alcohol.
- at least vinyl alcohol, (meth)allyl alcohol, isoprenol or methylbutenol are especially preferred possibilities as representatives.
- the ethylenically unsaturated monomer side groups of the component B) or C) can however also contain at least one C 4 oxyalkylene unit.
- At least one ethylenically unsaturated monomer of the components B) or C) can have a C 2 -C 8 carboxylate ester, which in particular is hydrolysable.
- the present invention includes a modification wherein the oxyalkyl side groups have at least one ethylene oxide, one propylene oxide, one polyethylene oxide, one polypropylene oxide or mixtures thereof.
- the copolymer a 2 ) in the component C) can have at least one nonionic (“uncharged”) and/or one non-hydrolysable monomer residue or mixtures thereof.
- the present invention also includes a third modification of the comb polymer a), which then is a nonionic (uncharged) copolymer a 3 ).
- a third modification of the comb polymer a which then is a nonionic (uncharged) copolymer a 3 ).
- Q stands for an ethylenically unsaturated monomer with at least one hydrolysable residue
- R 1 and R 2 mutually independently mean at least one C 2 -C 8 alkyl
- R 3 comprises (CH 2 ) c , where c is a whole number between 2 and 5 and where mixtures of the representatives of R 3 in the same polymer molecule are possible
- R 5 means at least one representative selected from the series H, a linear or branched, saturated or unsaturated C 1 -C 20 aliphatic hydrocarbon residue, a C 5 -C 8 cycloaliphatic hydrocarbon residue or a substituted or unsubstituted C 6 -C 14 aryl residue
- nonionic copolymer a 3 can alternatively also be a representative of the general formula (V),
- the hydrolysable residue can be at least one representative of the series alkyl ester, aminoalkyl ester, hydroxyalkyl ester, aminohydroxyalkyl ester or amide.
- the present invention specifies at least one representative of the general formula (VI)
- R 4 means at least one C 1 -C 20 alkyl or C 2 -C 20 hydroxyalkyl radical and the radicals G, p, R, R 1 , R 2 , R 3 , c, R 4 , R 5 and m, n, w, y, z and (y+z) have the meanings stated under the formulae (IV) and (V).
- the molar ratio of w to the sum (y+z) is 1:1 to 20:1 and preferably 2:1 to 12:1.
- the representative of the third modification of the copolymer a 3 ) corresponding to formula (VI) should in particular be a nonionic polyether-polyester copolymer.
- the present invention specifies that the formulation contain the component a) in proportions from 5 to 95% by weight, preferably of 10 to 60% by weight and particularly preferably of 15 to 40% by weight, based in each case on the total formulation.
- Sulphonic acid group containing s-triazines or naphthalene-formaldehyde condensates are broadly disclosed by prior art documents and frequently used as water reducing agents or plasticizers for cement based systems such as concrete.
- BNS ⁇ -naphthalene-sulphonate-formaldehyde condensates
- NFS naphthalene-formaldehyde sulphonates
- BNS or NFS is suitable for making cement particles with high dispersion, low foaming and high range water reducing and thereof it is possible to save the hydraulic binder such as cements or calcium sulphite based binders to improve the cement mobility and workability.
- BNS is a high range admixture for concrete, cast-in-place, prefabricating, pump and curing and BNS has a good adaptability to cements and other hydraulic binders and is not corrosive to reinforcing bar and non poisonous and pollution-free. Therefore it has been broadly applied to the construction industry such as highways, bridges, tunnels, industrial buildings, prestressing force components and high range concretes.
- condensates suitable as plasticizer or dispersants are prepared by the reaction of aromatic sulphonic acids like naphthalene sulphonic acid with formaldehyde under ambient pressure and under temperatures up to 100° C.
- BNS The preparation and use of BNS is well known state of the art and disclosed for example in EP 0 214 412 A1 and DE-PS 2 007 603.
- the effect and properties of BNS can be modified by changing the molar ratio between formaldehyde and the naphthalene component that usually is from 0.7 up to 3.5.
- the ratio between formaldehyde and the sulphonated naphthalene component preferably is from 0.8 to 3.5 to 1.
- BNS condensates are added to the hydraulic binder containing composition in amounts from 0.01 up to 6.0 wt. %.
- Melamine-sulphonate-formaldehyde-condensates are broadly used as flow improving agents in the processing of hydraulic binder containing compositions such as dry mortar mixtures, pourable mortars and other cement bonded construction materials.
- Melamine is used in this connection as representative of the s-triazine which is why these improving agents are known as MFS resins. They cause as well as the already mentioned BNS representatives a strong liquefying effect of the construction chemicals mixture without any undesired side effects occurring in the processing or in the functional properties of the hardened building material.
- MFS resins The liquefying effect of MFS products is achieved without lowering the surface tension of the water and binder system which usually is the case for the example with BNS products or flow improving agents with a surfactant-like polymers structure.
- the advantage of MFS resins is presumed to be due to the fact that no air avoids are introduced in to mortar during remixing process and the mortar density and strengths are not adversely effected after hardening.
- MFS resins provide the fresh mortar mixture with a good cohesive strength so that even when the flow properties are extreme separation phenomena within the construction composition do not occur. This phenomenon, also called as “segregation”, is feared especially in the production of self-flowing smoothing compositions which especially is the case with self-leveling screeds since its leads to a non-uniform layer structure of the screed due to floating of the fine material and sedimentation of the coarse grain.
- DE 196 09 614 A1 discloses a water soluble polycondensation product based on an amino-s-triazine and its use as plasticizer in aqueous binder containing suspensions based on cement, lime and gypsum. These polycondensates are capable in two condensation steps whereby in a pre-condensation step the amino-s-triazine, the formaldehyde component and the sulphite are condensated at a molar ratio of 1 to 0.5:5.0 to 0.1:1.5.
- Melamine is a preferred representative of amino-s-triazines. Further suitable representatives are amino plast former selected from the group urea, thiourea, dicyandiamide or guanidine and guanidine salts.
- sulfanilic acid containing condensation products based on amino-s-triazines that show at least two amino groups are prepared by using formaldehyde.
- the sulfanilic acid is used in amounts of from 1.0 to 1.6 mol per mol amino-s-triazine and neutralized in aqueous solution with an alkaline metal hydroxide or in earth alkaline metal hydroxide.
- the formaldehyde is added in amounts of from 3.0 to 4.0 mol per mol amino-s-triazine at a pH value between 5.0 to 7.0 and at temperatures between 50 and 90° C.
- the final viscosity of the solution shall be between 10 and 60 cSt at 80° C.
- DE 195 38 821 A1 discloses a condensate based on an amino-s-triazine with at least two amino groups and formaldehyde and a high content of sulphonic acid groups and a low content of formate.
- Such products can be prepared according to this document by reacting the amino-s-triazine, formaldehyde and a sulphite at a molar ratio of 1:3.0:6.0:1.51:2.0 in an aqueous solution and at a temperature between 60 and 90° C. and a pH value between 9.0 and 13.0 until the sulphite is no longer present.
- the condensation process is conducted at a pH value between 3.0 and 6.5 and at temperatures between 60 and 80° C.
- the BNS and/or MFS dispersant is used in amounts of from 0.01 to 10 wt. % and preferably 0.1 to 5 wt. %, related to the hydraulic binder component.
- the molar ratio of the sulphonic group and related to the melamine component is of from 1.0 to 2.0 and the molar ratio of the formaldehyde related to the melamine component is from 2.5 to 5.0.
- the molar ratio melamine to sulphonic acid to formaldehyde is 1:1.1:1.5:3.3:3.6.
- the molar ratio of formaldehyde to naphthalene sulphonic acid is from 1.3 to 1:3 to 1.
- admixtures in the form of dispersants are added to aqueous slurries or pulverulent inorganic or organic substances, such as clays, silicate powder, chalk, carbon black, crushed rock and hydraulic binders, for improving their processability, i.e. kneadability, spreadability, sprayability, pumpability or flowability.
- Such admixtures are capable of preventing the formation of solid agglomerates and of dispersing the particles already present and those newly formed by hydration and in this way improving the processability. This effect is utilized in particular in a targeted manner in the preparation of construction material mixtures which contain hydraulic binders, such as cement, lime, gypsum, hemihydrate or anhydrite.
- admixtures are used which are generally referred to as water-reducing agents or plasticizers.
- water-reducing agents or plasticizers.
- plasticizers In practice, in particular polycondensates and copolymers are used as such agents.
- WO 2006/042709 describes polycondensates based on an aromatic or heteroaromatic compound (A) having 5 to 10 C atoms or heteroatoms, having at least one oxyethylene or oxypropylene radical, and an aldehyde (C) selected from the group consisting of formaldehyde, glyoxylic acid and benzaldehyde or mixtures thereof, which result in an improved plasticizing effect of inorganic binder suspensions compared with the conventionally used polycondensates and maintain this effect over a longer period (“slump retention”).
- these may also be phosphated polycondensates.
- the phosphated monomers used are, however, relatively expensive since they have to be separately prepared and purified.
- the polycondensate contains a further structural unit (X) which is represented by the following formula
- R 5 and R 6 in structural unit (X), independently of one another, are preferably represented by H, COOM c and/or methyl.
- the molar ratio of the structural units (VII), (VII), (IX) and (X) of the phosphated polycondensate according to the invention can be varied within wide ranges. This has proved to be expedient if the molar ratio of the structural units [(VII)+(VII)+(IX)]:(X) is 1:0.8 to 3, preferably 1:0.9 to 2 and particularly preferably 1:0.95 to 1.2.
- the molar ratio of the structural units (VII):[(VIII)+(IX)] in component b) is usually 1:15 to 15:1, preferably 1:10 to 10:1 and more preferably 1:5 to 3:1.
- the molar ratio of the structural units (VIII):(IX) is adjusted to 1:0.005 to 1:10, preferably 1:0.01 to 1:1, in particular 1:0.01 to 1:0.2 and more preferably 1:0.01 to 1:0.1.
- the groups A and D in the structural units (VII), (VIII) and (IX) of the polycondensate are generally represented by phenyl, 2-hydroxyphenyl, 3-hydroxyphenyl, 4-hydroxyphenyl, 2-methoxyphenyl, 3-methoxyphenyl, 4-methoxyphenyl, naphthyl, 2-hydroxynaphthyl, 4-hydroxynaphthyl, 2-methoxynaphthyl, 4-methoxynaphthyl, preferably phenyl, it being possible for A and D to be chosen independently of one another and also in each case to consist of a mixture of said compounds.
- the groups B and E, independently of one another, are preferably represented by O.
- R 1 , R 2 , R 3 and R 4 can be chosen independently of one another and are preferably represented by H, methyl, ethyl or phenyl, particularly preferably by H or methyl and especially preferably by H.
- a in structural unit (VII) is preferably represented by an integer from 5 to 280, in particular 10 to 160 and particularly preferably 12 to 120 and b in structural units (VIII) and (IX) by an integer from 0 to 10, preferably 1 to 7 and particularly preferably 1 to 5.
- the respective radicals, the length of which is defined by a and b, respectively, may consist here of uniform building blocks, but a mixture of different building blocks may also be expedient.
- the radicals of the structural units (VII) or (VIII) and (IX), independently of one another may each have the same chain length, a and b each being represented by a number. As a rule, however, it will be expedient if mixtures having different chain lengths are present in each case so that the radicals of the structural units in the polycondensate have different numerical values for a and independently for b.
- the phosphated polycondensate according to the present invention has a weight average molecular weight of 4000 g/mol to 150 000 g/mol, preferably 10 000 to 100 000 g/mol and particularly preferably 20 000 to 75 000 g/mol.
- the phosphated polycondensate according to the invention is present in the claimed formulation as aqueous solution which contains 2 to 90% by weight of water and 98 to 10% by weight of dissolved dry matter, preferably 40 to 80% by weight of water and 60 to 20% by weight of dissolved dry matter, and more preferably 45 to 75% by weight of water and 55 to 25% by weight of dissolved dry matter.
- the dry matter then substantially comprises the anhydrous phosphated polycondensate, where further components, such as antifoams and other auxiliaries, can advantageously also be present.
- the invention furthermore envisages a sodium, potassium, ammonium and/or calcium salt and preferably a sodium and calcium salt, of the phosphated polycondensate.
- the present invention also relates to a process for the preparation of a phosphated polycondensate, it being regarded as essential that the polycondensation and the phosphation be carried out in a reaction mixture.
- This is to be understood as meaning that the phosphated component formed in the reaction solution needs neither be purified nor isolated.
- the phosphation can be carried out before, during or after the polycondensation. It is to be regarded as being preferred here to carry out both the phosphation and the polycondensation in the same reaction vessel.
- reaction mixture with regard to the polycondensate component b) contains at least
- the monomers (Ia), (IIa), (IIIa) and (IVa) and, in the polycondensate, the structural unit (IIa) are preferably represented by the following general formulae:
- the present invention provides different variants of the reaction procedure.
- One possibility consists in first reacting the monomer (IIIa) with a phosphating agent and subjecting the monomer (IIa) thus obtained to polycondensation with the monomers (Ia), (IIIa) and (IVa).
- the monomer (IIIa) may originate here from an incomplete reaction during the phosphation reaction or can be deliberately added to the reaction mixture after the phosphation reaction.
- the monomers (Ia), (IIIa) and (IVa) it is also possible to subject the monomers (Ia), (IIIa) and (IVa) to a polycondensation and then to react the polycondensate obtained with a phosphating agent.
- the monomers (Ia), (IIIa) and (IVa) and the phosphating agent are reacted simultaneously.
- polyphosphoric acid and/or phosphorous pentoxide have proved suitable here as phosphating agents.
- the polycondensation is carried out in the presence of an acidic catalyst, this preferably being sulphuric acid, methanesulphonic acid, para-toluenesulphonic acid or mixtures thereof.
- an acidic catalyst this preferably being sulphuric acid, methanesulphonic acid, para-toluenesulphonic acid or mixtures thereof.
- the polycondensation and the phosphation are advantageously carried out at a temperature between 20 and 140° C. and a pressure between 1 and 10 bar.
- a temperature range between 80 and 110° C. has proved to be expedient.
- the duration of the reaction may be between 0.1 and 24 hours, depending on temperature, the chemical nature of the monomers used and the desired degree of crosslinking. Once the desired degree of crosslinking has been reached, which can also be determined, for example, by measurement of the viscosity of the reaction mixture, the reaction mixture is cooled.
- the reaction mixture is subjected to a thermal aftertreatment at a pH between 8 and 13 and a temperature between 60 and 130° C. after the end of the condensation and phosphation reaction.
- a thermal aftertreatment which advantageously lasts for between 5 minutes and 5 hours, it is possible substantially to reduce the aldehyde content, in particular the formaldehyde content, in the reaction solution.
- the present invention envisages subjecting the reaction mixture to a vacuum aftertreatment at pressures between 10 and 900 mbar after the end of the condensation and phosphation reaction, for reducing the aldehyde content.
- a vacuum aftertreatment at pressures between 10 and 900 mbar after the end of the condensation and phosphation reaction, for reducing the aldehyde content.
- other methods known to the person skilled in the art for reducing the formaldehyde content may also be used.
- An example is the addition of small amounts of sodium bisulphite, ethylene urea and/or polyethylenimine.
- the phosphated polycondensates obtained by these processes can be used directly as component b).
- Sodium hydroxide, potassium hydroxide, ammonium hydroxide or calcium hydroxide has proved to be particularly expedient here, it being regarded as being preferred to neutralize the reaction mixture.
- other alkali metal and alkaline earth metal salts and salts of organic amine are suitable as salts of the phosphated polycondensates.
- the present invention also provides the preparation of mixed salts of the phosphated polycondensates.
- These can expediently be prepared by reacting the polycondensates with at least two basic compounds.
- salts of the polycondensates according to the invention with which the duration of the processability of aqueous suspensions of inorganic binders and in particular of concrete can be influenced. While a reduction in the processability over time is observable in the case of the sodium salt, a complete reversal of this behavior takes place in the case of the calcium salt of the identical polymer, a smaller water reduction (smaller slump) occurring at the beginning and increasing with time.
- the corresponding phosphated polycondensates which consist of sodium and calcium salts, are prepared by reaction with a mixture of basic calcium and sodium compounds, in particular calcium hydroxide and sodium hydroxide.
- the catalyst used can also be separated off. This can expediently be effected via the salt formed during the neutralization. If sulphuric acid is used as a catalyst and the reaction solution is treated with calcium hydroxide, the calcium sulphate formed can be separated off, for example, in a simple manner by filtration.
- the phosphated polycondensate can be separated from the aqueous salt solution by phase separation and can be isolated. The phosphated polycondensate can then be taken up in the desired amount of water.
- the claimed formulation contains additionally to the components a) and b) at least one antifoaming agent c) and/or a component d) having a surface-active effect, the components c) and d) being structurally different from one another.
- said antifoaming agent c) comprises tri-alkylphosphate and more preferably triiso-butylphosphate, a polyoxypropylen copolymer and a glycerol/alcohol acetate.
- the invention additionally comprises an admixture wherein said antifoaming agent c) comprises a mixtures of a tri-alkylphosphate and a polyoxypropylene copolymer.
- the second optional component of the formulation namely the surfactant, is preferably selected from the group consisting of a ethylene oxide/propylene oxide (EO/PO) block copolymer, a styrene/maleic acid copolymer, a fatty alcohol alkoxylate, an alcohol ethoxylate R 10 -(EO)—H with R 10 being an aliphatic hydrocarbon group having from 1 to 25 carbon atoms, acetylenic diols, monoalkylpolyalkylenes, ethoxylated nonylphenols, alkylsulfates, alkylethersulfats, alkylethersulfonates, alkyl ether carboxylates.
- EO/PO ethylene oxide/propylene oxide
- R 10 being an aliphatic hydrocarbon group having from 1 to 25 carbon atoms, acetylenic diols, monoalkylpolyalkylenes, ethoxylated nonylphenols, alky
- More preferably surfactant component d) comprises an alcohol having a polyalkylene group consisting of a carbon chain length of 2 to 20 carbon atoms, with a specific carbon chain length of C 3 -C 12 .
- the formulation according to the invention comprises an aqueous composition that contains the antifoaming agent component c) in free form or attached to the dispersing components a), and/or b).
- the antifoaming agent is attached to the dispersing components it can be physically or chemically attached, and the chemically attached in this case in polymerized and/or grafted form being preferred.
- the antifoaming agent c) also can be considered as a third co-monomer of the copolymeric dispersing components a 1 ), a 2 ), a 3 ).
- the antifoaming agent c) is a blend component of the formulation.
- antifoaming agent component c) is either physically and/or chemically attached to the dispersing components a 1 ), a 2 ) and/or a 3 ) and/or it is a free form component and therefore constituent of a blend.
- the antifoaming component c) is present in amounts of 0.01 to 10% by weight and/or the surface-active component d) is present in amounts of 0.01 to 10% by weight, based in each case on the total weight of the formulation.
- the antifoaming formulation according to any of Claims 52 to 61 characterized in that the antifoam c) and/or the surface-active component d), independently of one another, are present in each case in an amount of 0.01 to 5% by weight, based in each case on the total weight of the formulation.
- the present invention additionally comprises an embodiment whereby the formulation in addition to the components a) and b) and optionally c) and/or d), contains at least one further compound e) selected from the group consisting of a polymer having a low charge, a neutral polymer or polyvinyl alcohol.
- This component e) and particularly its specific role in systems containing calcium sulfate as hydraulic binder has been teached in the unpublished provisional European Patent application EP 08171022.0.
- the component e) plays a major role in gypsum composition with certain clay contents.
- a formulation based on a branched comb polymer with ethylene oxide (EO) units in the side-chains for the dispersion of clay-containing gypsum mixtures are capable of masking clay minerals such as are in particular contained in natural gypsum to a sufficient extent that the surfaces thereof are no longer available for the adsorption of dispersants. They have no adverse effect on the fluidization and consistency of the wet and unhardened gypsum mixture and they are stable to the temperatures used in the drying of the gypsum products, so that no odour problems arise.
- a copolymer component a 2 is to prefer that is based on a hydrolysable monomer A having an active binding site for at least one component of the clay-containing gypsum mixture.
- the surface of the clay particles can be more effectively coated through the bunching of flexible EO side-chains on a polymer backbone or the clay particles can themselves be better flocculated overall. Because of the lower charge density, the component e) can adsorb mainly on the clay and not on the binder such as gypsum hemihydrate.
- PO polyethylene oxide
- the mixed modifications can also each be implemented in at least one, that is the same, side-chain.
- the component e) optionally contained in the formulation according to the invention as a sacrificial substance to some extent differs only insignificantly from the dispersants a) commonly used in clay-containing gypsums, since it also consists inter alia of polycarboxylate ethers.
- the difference consists however in the charge state, since only representatives with low or neutral charge are possible as the sacrificial substance.
- the manufacture of gypsum products in particular can also be effected with the aid of dispersants which inter alia consist of copolymer mixtures wherein the low-charge or neutral polymer fractions predominantly mask the clay minerals and thus enable the remaining dispersant content to exert its actual fluidizing agent action.
- the advantageous action of the formulation according to the present invention and mainly based on component e) is displayed in essentially all clay-containing gypsum mixtures.
- the positive action is especially pronounced in gypsum systems which contain at least one representative of the series calcium sulphate, calcium sulphate semihydrate or calcium sulphate hemihydrate, anhydrite and gypsum.
- the clay fraction in the gypsum mixture should preferably be swellable and in particular water-swellable and derive from the series of the smectites, montmorillonites, bentonites, vermiculites, hectorites or from the series of the kaolins, feldspars and micas such as for example illite and mixtures thereof.
- the present invention recommends clay contents in the gypsum mixtures of ⁇ 10 wt. %, preferably ⁇ 6 wt. %, preferably ⁇ 4 wt. % and especially preferably between 0.5 and 3 wt. %, each based on the gypsum component.
- proportions from 0.01 to 0.40 wt. %, preferably from 0.02 to 0.30 wt. %, preferably from 0.03 to 0.15 wt. % and especially preferably from 0.5 to 0.10 wt. %, each again based on the gypsum component, are recommended.
- the formulation contains the component e) in amounts of 1 to 50% by weight, preferably of 5 to 40% by weight and particularly preferably in amounts of 10 to 30% by weight, based in each case on the total weight of the formulation.
- the polymer component e which reacts with the clay particles in the gypsum mixture, is of particular significance.
- this should be branched, the side-chain preferably consisting of a polyether.
- Polycarboxylate ethers and/or polycarboxylate esters, preferably with EO side-chains and with a carboxylate content up to 83 mol. %, and preferably up to 75 mol. % are to be regarded as especially preferred in this connection.
- component a) of the formulation should advantageously include at least one polycarboxylate derivative (ether, ester); in particular if this has a low charge content, it cannot on account of its specific properties adsorb for example onto gypsum to the necessary extent. For this reason, the generally known dispersant action of polycarboxylate ethers and esters in particular does not occur to the necessary extent in this case. Hence the content of the charge-bearing component is important for the dispersant action of such representatives.
- ether, ester polycarboxylate derivative
- copolymer components a 1 ), a 2 ) and a 3 ) and, to some extent, depending on its chemical character, also component e) can compete with one another as regards the dispersant action, it is advantageous overall to select the respective contents in the formulation according to the invention such that the copolymer component a) can exhibit its dispersant action to the maximum and the component e) because of its charge properties has as little dispersant action as possible, but instead is maximally adsorbed on the clay particles.
- a low-charge polymer with a polyether side-chain is used as component e), then this should be made up of at least one monomer selected from the series polyether monoacrylate, polyether monomethacrylate, polyether monoallyl ether, polyether monomaleate, monovinylated polyether or mixtures thereof.
- this can be an alkylene oxide polymer with a molecular weight from 500 to 10 000, preferably from 750 to 7500 and in particular from 1000 to 5000.
- alkylene oxide polymers those based on an ethylene oxide, propylene oxide, butylene oxide or mixtures thereof may be mentioned.
- Low-charge polymers which are built up of at least one monomer selected from the series polypropylene glycol acrylates, polypropylene glycol methacrylates, polyethylene glycol acrylates, polyethylene glycol methacrylates, polypropylene glycol monovinyl ethers, polythylene glycol monovinyl ethers, alkoxy or aryloxypolyethylene glycol acrylates, alkoxy or aryloxypolyethylene glycol methacrylates, alkoxy or aryloxy-polyethylene glycol monovinyl ethers, acrylates, methacrylates and monovinyl ethers of an oxyethylene and oxypropylene block or randomized copolymer, polypropylene glycol allyl ether, polyethylene glycol allyl ether, polyethylene glycol monomaleate, polypropylene glycol monomaleate and any mixtures thereof have been found especially suitable.
- the polymer e) having a low charge carries a carboxylic acid group, preferably selected from the series consisting of acrylic acid, methacryl acid, maleic acid, fumaric acid, itaconic acid or anhydrides thereof.
- the low-charge polymer can also bear a carboxylic acid and/or sulphonic acid groups.
- the carboxylic acid group is preferably at least one representative of the series acrylic acid, methacrylic acid, maleic acid, fumaric acid, itaconic acid or anhydrides thereof.
- 2-Acrylamido-2-methylpropanesulphonic acid (AMPS) vinylsulphonic acid, allyl ether sulphonic acid, 2-sulphoethylmethacrylic acid, styrenesulphonic acid, methallyl-sulphonic acid, and sodium, potassium and ammonium salts and any mixtures thereof, are preferred representatives of compounds which make sulphonic acid groups available.
- AMPS and vinylsulphonic acid are to be regarded as especially preferable.
- neutral polymers as component e), these should be made up of neutral monomer building blocks, which are in particular selected from the series acrylic acid alkyl esters and methacrylic acid alkyl esters and hydroxyalkyl esters thereof with up to 5 carbon atoms. Particularly suitable in this case are hydroxyethyl acrylate and hydroxypropyl acrylate and hydroxyethyl methacrylate and hydroxypropyl methacrylate. Also possible are vinyl acetate, N-vinylpyrrolidone, N-vinylcaprolactam, styrene and methylstyrene.
- the present invention relates to a formulation that contains as additional further component f) a calcium-silicate-hydrate (C-S-H) containing composition.
- C-S-H calcium-silicate-hydrate
- admixtures for building material mixtures comprising hydraulic binders typically also contain hardening accelerators which shorten the setting time of the hydraulic binder.
- hardening accelerators which shorten the setting time of the hydraulic binder.
- C-S-H calcium silicate hydrate
- commercially available C-S-H or corresponding C-S-H dispersions may be regarded only as hardening accelerators which have little effect.
- the C-S-H containing composition is prepareble by reaction of a water-soluble calcium containing compound with a water-soluble silicate containing compound, the reaction of the water-soluble calcium containing compound with the water-soluble silicate containing compound being carried out in the presence of an aqueous solution preferably containing a water-soluble copolymer that preferably is a dispersant for hydraulic binders and selected from at least a representative of component a) and/or b).
- water-soluble calcium compounds and water-soluble silicate compounds are also suitable in each case as water-soluble calcium compounds and water-soluble silicate compounds, although readily water-soluble compounds (which dissolve completely or virtually completely in water) are preferred in each case.
- a reactivity sufficient for the reaction is present in an aqueous environment with the corresponding reactant (either water-soluble calcium compound or water-soluble silicate compound). It is probably to be assumed that the reaction takes place in aqueous solution but a water-insoluble inorganic compound (C-S-H) is usually present as a reaction product.
- comb polymers are to be understood as meaning those polymers which have relatively long side chains (having a molecular weight of in each case at least 200 g/mol, particularly preferably at least 400 g/mol) on a linear main chain at more or less regular intervals.
- the lengths of these side chains are frequently approximately equal but may also differ greatly from one another (for example when polyether macromonomers having side chains of different lengths are incorporated in the form of polymerized units).
- component f) acts as accelerator and in a preferred embodiment contains an inorganic and an organic component.
- the inorganic component may be regarded as modified, finely disperse calcium silicate hydrate (C-S-H) which may contain foreign ions, such as magnesium and aluminium.
- C-S-H can be prepared in the presence of the comb polymer plasticizer (organic component).
- a suspension containing the C-S-H in finely disperse form is obtained, which suspension firstly acts as a plasticizer and secondly effectively accelerates the hardening process of hydraulic binders.
- the inorganic component can in most cases be described with regard to its composition (not with regard to particle size, specific surface area, etc) by the following empirical formula:
- 0.1 ⁇ a ⁇ 2 preferably 0.66 ⁇ a ⁇ 1.7 0 ⁇ b ⁇ 1 preferably 0 ⁇ b ⁇ 0.1 1 ⁇ c ⁇ 6 preferably 1 ⁇ c ⁇ 6.0 0 ⁇ d ⁇ 1 preferably 0 ⁇ d ⁇ 0.4 0 ⁇ e ⁇ 2 preferably 0 ⁇ e ⁇ 0.1
- the C-S-H shows a calcium/silicium (Ca/Si)-molar ratio of 0.5 to 2.0, preferable 0.7 to 1.8, more preferable 1.6 to 1.7.
- the average particle size of C-S-H is smaller than 10 ⁇ m, preferable smaller than 1 ⁇ m, more preferable smaller than 0.2 ⁇ m, measured by light scattering with the equipment Master Sizer 2000 from the Malvern Company.
- the average particle size of C-S-H is greater 0.01 ⁇ m, preferable 0.1 ⁇ m to 1.0 ⁇ m, more preferable 0.2 ⁇ m to 0.5 ⁇ m.
- the dispersant that is used for this method of preparation can be identical to the representatives of the dispersing component a) and/or b) of the formulation.
- the dispersing agent in this method of preparation is necessary for achieving a small particle size distribution of the C-S-H compound.
- C-S-H containing composition is preperable by reaction of a calcium oxide, a calcium carbonate and/or a calcium hydroxide with a silicium dioxide during milling, the reaction being carried out in the presence of an aqueous solution that preferably contains a water-soluble copolymer that preferably is a dispersant for hydraulic binders and selected from at least a representative of component a) and/or b).
- the water-soluble calcium compound is mixed in a first step, with the aqueous solution which contains a water-soluble comb polymer suitable as a plasticizer for hydraulic binders, so that a mixture preferably present as a solution is obtained, to which the water-soluble silicate compound is added in a subsequent second step.
- the aqueous solution may also contain one or more further solvents in addition to water.
- the aqueous solution containing the dispersant and preferably one that is selected from component a) and/or b) furthermore has the water-soluble calcium compound and the water-soluble silicate compound as components dissolved in it.
- the aqueous solution also contains, in addition to silicate and calcium ions, further dissolved ions which are preferably provided in the form of dissolved aluminium chloride and/or dissolved magnesium chloride.
- the water-soluble dispersant can be a comb polymer and be present as a copolymer which contains, on the back bone, side chains having ether functions and acid functions.
- the water-soluble comb polymer is present as a copolymer which is produced by free radical polymerization in the presence of acid monomer and polyether macromonomer, so that altogether at least 45 mol %, preferably at least 80 mol %, of all structural units of the copolymer are produced by incorporation of acid monomer and polyether macromonomer in the form of polymerized units.
- Acid monomer is to be understood as meaning monomers which are capable of free radical copolymerization, have at least one carbon double bond, contain at least one acid function and react as an acid in an aqueous medium.
- acid monomer is also to be understood as meaning monomers which are capable of free radical copolymerization, have at least one carbon double bond, form at least one acid function in an aqueous medium as a result of a hydrolysis reaction and react as an acid in an aqueous medium (example: maleic anhydride).
- polyether macromonomers are compounds which are capable of free radical copolymerization, have at least one carbon double bond, and have at least two ether oxygen atoms, with the proviso that the polyether macromonomer structural units present in the copolymer have side chains which contain at least two ether oxygen atoms.
- the water-soluble calcium compound is present as calcium chloride, calcium nitrate, calcium formate, calcium acetate, calcium bicarbonate, calcium bromide, calcium carbonate, calcium citrate, calcium chlorate, calcium fluoride, calcium gluconate, calcium hydroxide, calcium hypochloride, calcium iodate, calcium iodide, calcium lactate, calcium nitrite, calcium oxalate, calcium phosphate, calcium propionate, calcium silicate, calcium stearate, calcium sulphate, calcium sulphate hemihydrate, calcium sulphate dihydrate, calcium sulphide, calcium tartrate and/or calcium aluminate, tricalcium silicate and/or dicalcium silicate.
- the water-soluble calcium compound is preferably present as calcium chloride, calcium nitrate and/or calcium formate.
- the water-soluble silicate compound is present as sodium silicate, potassium silicate, waterglass, aluminium silicate, tricalcium silicate, dicalcium silicate, calcium silicate, silicic acid, sodium metasilicate and/or potassium metasilicate.
- the water-soluble silicate compound is preferably present as sodium metasilicate, potassium metasilicate and/or waterglass.
- a calcium silicate (provided that it is soluble) may be used both as a silicate source and as a calcium source. In many cases, however, this is not preferred. As a rule, species of different types are used as the water-soluble silicate compound and as the water-soluble calcium compound.
- the formulation is a liquid or a powder and preferably a redispersant powder.
- the powder form of the formulation can be achieved by any method known to a skilled person. Preferred is the spray drying method that is also suitable for getting the formulation of the invention as redispersant powder.
- the use of the formulation for controlling the flowability of aqueous suspensions used in construction chemistry and in particular in aqueous suspensions containing hydraulic and/or latent hydraulic binders is of main interest.
- the formulation is used in particular as composition with dispersing properties.
- these compositions contain, as a hydraulic binder at least one representative selected from the group consisting of cements and calcium sulphate-based compounds, in particular calcium sulphate hemihydrate, anhydrite or gypsum.
- the aqueous suspension according to the present invention preferably is based on a dry mortar composition or a flooring composition.
- the flooring composition contains calcium sulphate or cement or mixtures thereof, and preferably is a self-leveling flooring composition.
- the formulation according to the present invention is to be used in amounts of 0.001 to 8.0% by weight, in particular 0.005 to 5.0% by weight, preferably 0.01 to 2.0% by weight and particularly preferably 0.05 to 1.0% by weight, based in each case on the total composition of the suspension.
- the present invention comprises the option that the formulation is used together with other admixtures or compositions, preferably with flowability controlling and/or dispersing properties, and more preferably together with at least one dispersant of the type of component a) and/or the polymerisation product b) of the formulation.
- component a) and component b) can be used as formulation according to the present invention and additionally that this formulation can be used together with other compounds, additives, admixtures or compositions.
- components a) and b) can be used as substantial constituents of the formulation and additionally as single compounds together with such formulation.
- This kind of use can be practiced stepwise, that means that either the formulation or the additional dispersants are added to the hydraulic binder containing composition in the first step of use and that additional amounts of the formulation its components are added in up following process steps.
- a reactor equipped with a stirrer and a heating mantle is filled with 600 parts of poly(ethyleneoxide)monophenylether (average molecular weight 5000 g/mol), 47.2 parts of concentrated methane sulfonic acid, 12 parts of water, 110 parts of ⁇ -phenyl- ⁇ -hydroxypoly(oxy-1,2-ethanediyl)phosphate (average molecular weight 368 g/mol) and 14.7 parts of paraformaldehyde.
- This reaction mixture is stirred at 115° C. for 3 h. After cooling, 830 parts of water are added the reaction mixture is neutralized with 50% sodium hydroxide solution to a pH value of 6.5 to 7.
- the resin is a light yellow colored, clear and aqueous polymer solution with a solid concentration of 40% by weight.
- the amounts of the materials shown in Table 2 are in percent by weight of the solution.
- a reactor equipped with a stirrer and a heating mantle is filled with 26 parts of polyphosphoric acid and heated to 90° C. Within 15 min 44.2 parts of 2-phenoxyethanol are charged into the reactor. After 1 h, 400 parts of poly(ethyleneoxide)monophenylether (average molecular weight 5000 g/mol), 31.4 parts of concentrated methane sulfonic acid, 20 parts of water and 12.6 parts of paraformaldehyde are added. This reaction mixture is stirred at 105° C. for 6 h. After cooling, 550 parts of water are added and the reaction mixture is neutralized with 50% sodium hydroxide solution to a pH value of 6.5 to 7.
- the resin is a light brown colored, clear and aqueous polymer solution with a solid concentration of 40% by weight.
- the amounts of the materials shown in Table 2 are in percent by weight of the solution.
- a reactor equipped with a stirrer and a heating mantle is filled with 51.6 parts of polyphosphoric acid and heated to 90° C. Within 15 min 90 parts of 2-phenoxyethanol are charged into the reactor. After 1 h, 322 parts of poly(ethyleneoxide)monophenylether (average molecular weight 5000 g/mol), 300 parts of poly(ethyleneoxide)monophenylether (average molecular weight 2000 g/mol), 42.1 parts of concentrated methane sulfonic acid, 16.8 parts of water and 28.5 parts of paraformaldehyde are added. This reaction mixture is stirred at 105° C. for 6 h.
- the reaction mixture is neutralized with 50% sodium hydroxide solution to a pH value of 6.5 to 7.
- the resin is a light brown colored, clear and aqueous polymer solution with a solid concentration of 40% by weight.
- the amounts of the materials shown in Table 2 are in percent by weight of the solution.
- the examples E1 till E20 were prepared by mixing the polycondensate components b) with equivalent amounts (wt. %) of the dispersants a).
- Melflux PCE 239 L 35% N.D., Melflux 2500 L 45% N.D., Melflux 2453 L 44% N.D., VP2661/493 L 40% N.D., Melflux 2424 L 50% N.D., Melflux AP 120 L 40%, and Sokalan DS5009 X are a polycarboxylate ether dispersant available from BASF Construction Polymers GmbH, Germany.
- Melcrete 500 L is a naphthalene sulfonate dispersant (BNS) available from BASF Construction Polymers GmbH.
- Melment L 15 G is a melamine sulphonate-formaldehyde condensate (MFS) available from BASF Construction Polymers GmbH.
- the non-ionic polymers N1 and N2 are able to maintain the fluidity of a cement composition and are synthesized according to the still unpublished application U.S. Ser. No. 12/477,637.
- antifoaming agent A1 has been a polypropyleneglycol commercially available as Pluriol® P2000 and, antifoaming agent A2 an alkoxylated alcohol commercially available as Degressal® SD23 and antifoaming agent A3 a carboxylic ester commercially available as Degressal® SD30 all from BASF SE (Ludwigshafen, Germany).
- Surfactant S1 was an ethoxylated oxo-alcohol commercially available as Lutensol® TO6 from BASF SE (Ludwigshafen, Germany).
- Surfactant S2 (as component a) is a styrene/maleic acid copolymer which was synthesized according to EP 0306449 A2.
- the viscosities of the dispersants solutions were measured with a capillary viscometer at 25° C.
- the solutions were prepared by mixing a 25 Wt. % BNS solution with 25 Wt. % solutions of the dispersants as indicated in Table 1 and 3.
- the viscosity of the admixture E3, E4 and E5 is only slightly higher compared to the viscosity of the pure condensates C7, C8 and C9. Whereas the admixtures E3, E4 and E5 keep their low viscosity over time, the mixture of BNS and the polycarboxylate ether starts to form a non pourable gel. Unlike C4, the admixture E3, E4 and E5 are usable as dispersant agents for hydraulic binders (see below for application tests).
- the required amount of liquid admixture was weighted into the mixing cup and water was added to reach the water to stucco ratios given in Table 4.
- the stucco (400 g from various sources) together with the accelerator is sifted into water within 15 sec and afterwards mixed with a Hobart® mixer for 15 sec at high speed (285 rpm). After 60 sec the flow value was measured with a cylinder (height: 10 cm, diameter: 5 cm). The set time was determined by means of the so-called knife cut test.
- the admixture E26 according to the invention show excellent dispersant abilities in comparison to the polycondensate dispersant C8.
- Admixture E31 which contains the polycondensate C8 and the polycarboxylate ether C2, displays a lower amount of accelerator usage as the pure polycarboxylate ether C2.
- the admixtures E7-1 and E7-2 according to the invention display excellent dispersant abilities in FGD stucco and in comparison to the polycarboxylate ether dispersant C3 a significant reduced accelerator demand.
- Admixtures E8-1 and E8-2 show higher flow values than the pure polycondensate C8.
- the admixture according to the invention E26 and E13 possesses fluidity in the natural stucco B.
- the comparison admixture C3 is not fluid whereas C2 needs a significant higher water to stucco ratio to reach the same flow value.
- the admixtures according to the invention display excellent dispersant abilities in the natural stucco C and in comparison to the polycarboxylate ether dispersant C3 reduced accelerator demands.
- the admixtures E26 and E15 have a similar low accelerator demand but a higher fluidity as the polycondensate C8.
- 600 g of cement powder is homogenized in a RILEM-Mixer.
- the required amount of water to reach the water to cement ratios given in Table 9 is added and mixed in for 30 sec at 140 rpm (level I).
- the sand is added during agitation via a funnel and mixed in for 30 sec at 140 rpm (level I).
- the brims of the bowl will be cleaned and the required amount of liquid admixture is added after a mixing break of 1.5 min.
- the mixing is continued for 60 sec at 285 rpm (level II) and afterwards the flow value (spread value) is determined with a Hagermann cylinder according to DIN EN 1015-3.
- the mortar is based on a Karistadt CEM I 42.5 R and has a sand to cement ratio of 2.2.
- the sand consists of a mixture of 30% quartz sand and 70% standardized sand.
- the admixture E16 that contains the low-cost dispersant BNS, displays similar fluidity as the condensate C7 at a much lower dosage level than BNS. As FIG. 9 shows, the admixtures E18 and in particular E13 and E20 keep the fluidity of the cementious binder composition for more than 90 min.
Abstract
Claimed is a formulation containing a) at least one component having dispersing properties and being selected from branched comb polymers having polyether side chains, a naphthalene sulphonate-formaldehyde condensate and a melamine sulphonate-formaldehyde condensate, and b) a polycondensation product. Typical representatives of component a) are polycarboxylate ether, polycarboxylate ester and uncharged copolymers. In addition to the main components a) and b) further additives such as anti-forming agents and tensides or polymers having a low charge, neutral polymers or polyvinyl alcohol can be comprised by the formulation that is suitable for controlling the flowability of aqueous suspensions of construction chemicals.
Description
- This application is related to co-pending U.S. Ser. No. 12/______, filed concurrently herewith and entitled “Gypsum Products Using Additives”, incorporated herein by reference in its entirety.
- The subject of the present invention is a formulation for the dispersion of hydraulic binder and especially gypsum containing compositions.
- Conventional dispersants for cementitious and gypsum compositions typically achieve good water reduction, however, they are limited in their ability to retain workability over a long period of time. An alternate method for extended workability retention is the use of retarding admixtures. In this scenario, the benefit of workability retention is often achieved at the expense of setting times and early strength. The usefulness of these dispersants is therefore limited by their inherent limitations in molecular architecture.
- Usual dispersants are static in their chemical structure over time in hydraulic systems. Their performance is controlled by monomer molar ratio that is fixed within a polymer molecule. A water reducing effect or dispersing effect is observed upon dispersant adsorption onto the hydraulic particle surface. As dispersant demand increases over time due to abrasion and hydration product formation, which creates more surface area, these conventional dispersants are unable to respond and workability is lost.
- Typically, the issue of extended workability is solved by either re-tempering (adding more water) to the hydraulic compositions or by adding more high range water reducer. Addition of water leads to lower strength and thus creates a need for mixes that are “over-designed” in the way of hydraulic binder content.
- Various types of organic compounds have been used to advantageously alter certain properties of wet hydraulic binder compositions. One class of components, which can collectively be called “superplasticizers” fluidify or plasticize wet binder compositions to obtain a more fluid mixture. A controlled fluidity is desired, such that the aggregate used in mortars and concretes does not segregate from the binder paste. Alternatively, superplasticizers may allow the cement composition to be prepared using a lower water: binder ratio in order to obtain a composition having a desired consistency which often leads to a hardened composition having a higher compressive strength development after setting.
- A good superplasticizer should not only fluidify the wet hydraulic binder composition to which it is added, but also maintain the level of fluidity over a desired period of time. This time should be long enough to keep the wet composition fluid, e. g. in a ready-mix truck while it is on its way to a job site. Another important aspect relates to the period for discharging the truck at the job site and the period needed for the cement composition for being worked in the desired final form. On the other side, the hydraulic mixture cannot remain fluid for a too long time, that means the set must not greatly be retarded, because this will slow down the work on the job and show negative influences on the characteristics of the final hardened products.
- Conventional examples of superplasticizers are melamine sulfonate/formaldehyde condensation products, naphthalene sulfonate/formaldehyde condensation products and lignosulfonates, polysaccharides, hydroxycarboxylic acids and their salts and carbohydrates.
- In most cases, fluidizing agents are multi-component products with copolymers based on oxyalkylenglykolalkenylethers and unsaturated dicarboxylic acid-derivatives as most important species. The European Patent EP 0 736 553 B1 discloses such copolymers comprising at least three sub-units and especially one unsaturated dicarboxylic acid derivative, one oxyalkylenglykolalkenylether and additionally one hydrophobic structural unit, such as ester units. The third structural unit can also be represented by polypropylenoxid- and polypropylenoxid-polyethylenoxid-derivatives, respectively.
- The German published application DE 195 43 304 A1 discloses an additive for water containing mixtures for the construction field comprising a) a water-soluble sulfonic acid-, carboxylic- or sulfate group containing cellulose derivative, b) a sulfonic acid- and/or carboxylic acid containing vinyl-(co)-polymer and/or a condensation product based on aminoplast-builders or acryl containing compounds and formaldehyde. This additive shall show sufficient water retention ability and rheology-modifying properties. Therefore, this additive shall be suitable for construction chemical compositions containing cement, lime, gypsum, anhydrite and other hydraulic binder components.
- Copolymers based on unsaturated monocarboxylic or dicarboxylic acid derivatives, oxyalkylenglykolalkenylethers, vinylic polyalkylenglykol, polysiloxane or ester compounds used as additives for aqueous suspensions based on mineral or bituminous binders are described in U.S. Pat. No. 6,777,517 B1. The use of such additives results in a decrease in the water/binder ratio and leads to highly fluid building materials without segregation of individual constituents from the building material mixture. The copolymers according to this U.S. patent are useful as additives for aqueous suspensions of inorganic and organic solids and especially for suspensions that are based on mineral or bituminous binders such as cement, plaster of paris, lime, anhydrite or other building materials based on calcium sulfate.
- Disclosed by prior art also are copolymers of ethylenically unsaturated ethers that can be used as plasticizers for cement containing mixtures (EP 0 537 870 A1). These copolymers contain an ether co-monomer and as additional co-monomer an olefinic unsaturated mono-carboxylic acid or an ester or a salt thereof, or alternatively an olefinic unsaturated sulfuric acid. These copolymers show a very short ether side chain with 1 to 50 units. The short side chain causes a sufficient plasticizing effect of the copolymers in cement containing masses with a reduced slump loss of the construction chemicals mass itself.
- U.S. Pat. No. 6,139,623 B1 discloses an emulsion admixture for use in hydraulic cement compositions formed by emulsifying an antifoaming agent, a surfactant and a copolymer having a carbon-containing backbone to which are attached groups that function as cement-anchoring members by forming ionic bonds and oxyalkylene groups. This admixture comprising an ethylene oxide/propylene oxide (EO/PO) type comb polymer and an antifoaming agent allows a predictable air control in hydraulic cement compositions such as concrete. The term “cement composition” refers to pastes, mortars, grouts such as oil well cementing grouts, and concrete compositions comprising a hydraulic cement binder. Typical antifoaming agents are phosphate ester, borate ester and polyoxyalkylene copolymers with defoaming properties. The surface active component (surfactant) is said to stabilize the emulsion mixture and is chosen from the group consisting of an esterified fatty acid ester of a carbohydrate, a C2 to C20 alcohol having polyoxyalkylene groups or a mixture thereof.
- US 2006/0281886 discloses a co-polymer comprising two monomer components with a component a) being an olefinic unsaturated monocarboxylic acid co-monomer or an ester or a salt thereof or an olefinic unsaturated sulfonic acid co-monomer or a salt thereof, and with component b) preferably represented by an ether compound. These two monomeric co-polymer can be preferably used as a superplasticizer in a hydraulic binder containing composition. There it is alternatively disclosed that the co-polymer can be used in combination with a defoaming component that is also an additional structural unit of the co-polymer. Consequently, the defoaming component can be chemically attached to the co-polymer or being present in free form in a blend. Under general aspects the prior art teaches the use of dispersing agents (plasticizers) such as polycarboxylate ethers (PCE) as typical additive for calcium sulfate containing binder systems. This results in a water reduction as well as in an enhancement of physical properties such as compressive strength. Additionally, the workability and preferably the rheological behavior of the construction chemicals composition are improved. On the other hand the addition of PCE based dispersants causes a distinct air entrainment to the binder component that worsens the physical properties of the composition. Another negative aspect is the foam formation during the preparation of the binder system. For overcoming these drawbacks defoamer components are used as additional additive to the dispersing agent. However, defoamers show a low solubility in aqueous formulations and cause an insufficient stability. Moreover, the defoaming properties of the formulation decrease over time due to the resulting phase separation of the defoamer and the dispersant.
- Based on the different characteristics and the availability of the superplasticizers mentioned above, it has been further desired to come up with new formulations suitable as admixtures which are an improvement over the current state of the art. It is thus an object of this invention to provide new formulations for calcium sulfate binder containing compositions which impart to wet binder compositions excellent fluidizing and water reduction properties. Furthermore, the properties, the performance and effects of the provided copolymer shall be arbitrary.
- In the production of gypsum plasterboard, in order to decrease the drying costs it is necessary to establish as low as possible a water/gypsum value. In addition, the gypsum mixture should set as rapidly as possible, so that the necessary cutting strength of the plate is attained on the conveyor line after as short a time as possible. For these reasons, dispersants based in particular on polycarboxylate ethers were developed (DE 10 2006 027 035 A1; U.S. Pat. No. 7,070,648 B1).
- US 2008/017078 teaches a liquid admixture composition for a calcium sulfate based binder system and a method of use. The disclosed admixture comprises an aqueous composition of a copolymeric dispersing component, an antifoaming agent component, a surfactant component and water. The components may be a blend or physically or chemically attached and result in a stable liquid system that can be used as dispersing agent for calcium sulfate compound containing construction chemicals composition. The admixture composition disclosed in this document and especially its application as dispersing agent represent a further improvement of this state of the art because the admixture with its contained aqueous composition induces a uniform plasticizing effect all the time and an improvement of the physical properties due to reduction of both water and air content in the wet construction chemicals gypsum mass. Furthermore, the admixture shows an improved storage stability and homogeneity.
- Gypsum mixtures for foaming, solid and fast drying gypsum products and a method of making a gypsum slurry by using modifiers and dispersants are disclosed by US 2009/0101045, US 2006/0281837, US 2006/0280899, US 2006/0280898, US 2006/0278135, US 2006/0278134, US 2006/0278130, US 2006/0278127, US 2005/0250888, US 2005/0239924 and US 2006/0280970. The dispersants mentioned in these documents represent polycarboxylate dispersants, the dispersant having two repeating units with an olefinic unsaturated mono-carboxylic acid repeating unit and a vinyl or allyl-group bound to a polyether by an ether linkage as second repeating unit.
- The results given in any of these documents confirm that such dispersants can be used to attain advantageous physical properties known from superplasticizers such as polycarboxylate ethers.
- It was therefore the object of the present invention to provide an economical and effective new formulation based on suitable and well established dispersing components for hydraulic binders, which dispersant is particularly suitable as a plasticizer/water reducing agent for concrete and other hydraulic binder based systems and that can be prepared in a simple manner and at low costs.
- Provided by this invention therefore is a formulation for extending workability to a hydraulic binder and preferably a calcium sulfate containing mixture and water, comprising introducing into the mixture a combination of dispersing components. The subject formulation achieves a better workability and fluidibility of hydraulic setting compositions and establishes a low water/hydraulic binder value.
- The present invention relates to a formulation containing
-
- a) at least one component having dispersing properties and selected from the group consisting of a compound at least containing a branched comb polymer having polyether side chains, a naphthalene sulphonate-formaldehyde condensate (“BNS”) and a melamine sulphonate-formaldehyde condensate (“MSF”), and
- b) a polycondensation product containing
- (I) at least one structural unit with an aromatic or heteroaromatic sub-unit and a polyether side chain, and
- (II) at least one phosphated structural unit with an aromatic or heteroaromatic sub-unit, and
- (III) at least one structural unit with an aromatic or heteroaromatic sub-unit,
- with structural unit (II) and structural unit (III) differing exclusively in that the OP(OH)2 group of the structural unit (II) is replaced by H in structural unit (III), and structural unit (III) is not the same as structural unit (I), the formulation being suitable as admixture for a hydraulic binder and preferably a calcium sulfate binder system containing composition.
- The term “hydraulic binder” according to this invention means cement and preferably Portland cement represented by CEM I, CEM II, CEM III, CEM IV and CEM V, white cement, quick lime and aluminate cement.
- The term “latent hydraulic binder” according to this invention means at least one representative selected from the group fly ash, blast furnace slag, metakaoline, microsilica, trass compounds, alumosilicates, tuff, phomulithe, diatomaceous earth and oil shell.
- The term “calcium sulfate compound” according to this invention means calcium sulfate in its anhydrous and hydrate forms, such as gypsum, anhydrite, calcium sulfate dihydrate and calcium sulfate hemi-hydrate.
- The term “gypsum” according to this invention is also known as calcium sulfate, whereby calcium sulfate can be used in its various anhydrous and hydrate forms with or without crystal water. Natural gypsum is represented by calcium sulfate dihydrate and the natural crystal water free form of calcium sulfate is represented by the term “anhydrite”. Besides the natural forms, calcium sulfate is a typical by-product of technical processes characterized by the term “synthetic gypsum”. One example of such technical processes is the flue gas desulphurization. Synthetic gypsum may also be a by-product of phosphorous acid and hydrogen fluoride production methods for gaining hemi-hydrate forms (CaSO4½H2O). Gypsum (CaSO4.2H2O) may be calcinated by driving off the water of hydration. Products of the various calcinating procedures are alpha or beta hemi-hydrate. Beta calcium sulfate hemi-hydrate results from a rapid heating in open units by a rapid evaporation of water and by forming cavities. Alpha hemi-hydrate is produced by a de-watering of gypsum in closed autoclaves. The crystal form in this case is dense and therefore, this binder needs less amounts of water than beta hemi-hydrate. On the other hand, gypsum hemi-hydrate re-hydrates with water to dihydrate crystals. Usually, the hydration of gypsum needs some minutes to hours indicating a clearly shortened workability period in contrast to cements that hydrate in periods over hours or days. These characteristics make gypsum an attractive alternative to cement as hydraulic binder in various fields of application, because hardened final gypsum products show a characteristic hardness and compressive strength.
- Calcium sulfate hemi-hydrate can produce at least two crystal forms, whereby α-calcined gypsum is usually de-watered (de-hydrated) in closed autoclaves. For various fields of application, β-calcined gypsum may be selected due to its availability under economical aspects. However, these advantages may be reversed because β-calcined gypsum needs higher water amounts for workability and for making slurries of a given fluidity. Hardened or dried gypsum tends to a certain weakening based on the remained water in its crystal matrix. Therefore, products thereof show less strength than gypsum products that have been made with smaller amounts of water.
- In general, the workability of gypsum, but also of other hydraulic binders, can be improved under hydraulic aspects by adding dispersants. In this connection, the formulation according to this invention represents a suitable dispersant because of the dispersing properties of its component.
- Component a) of the formulation according to the invention has dispersing properties and is selected from the group consisting of a compound at least containing a branched comb polymer having polyether side chains, a naphthalene sulphonate-formaldehyde condensate (“BNS”), and a melamine sulphonate-formaldehyde condensate (“MSF”),
- Formulations which contain a branched comb polymer having polyether side chains as the component a) with dispersant action have been found extremely effective. It therefore can be seen as preferred embodiment that the component a) is a polycarboxylate ether a1), a polycarboxylate ester a2), an uncharged copolymer a3) or a mixture thereof. In general and additionally to the dispersing properties of component a) polycarboxylate ester a2) are preferred that show anti-foaming and surface active activities.
- 1.1 Copolymer a1:
- Such polyether-containing copolymers, which in the sense of the present invention are suitable as component a1), have been previously described in WO 2006/133933 A2. These copolymers consist of two monomer components, the first monomer component being an olefinically unsaturated monocarboxylic acid comonomer or an ester or a salt thereof and/or an olefinically unsaturated sulphonic acid comonomer or a salt thereof, and the second monomer component a comonomer of the general formula (I)
- wherein R1 represents
- and R2 represents H or an aliphatic hydrocarbon residue with 1 to 5 C atoms; R3=unsubstituted or substituted aryl residue and preferably phenyl, and R4=H or an aliphatic hydrocarbon residue with 1 to 20 C atoms, cycloaliphatic hydrocarbon residue with 5 to 8 C atoms, a substituted aryl residue with 6 to 14 C atoms or a member of the series
- wherein R5 and R7 each represent an alkyl, aryl, aralkyl, or alkaryl residue and R6 for an alkylidene, arylidene, aralkylidene or alkarylidene residue, and
- p=0, 1, 2, 3 or 4
- m, n mutually independently mean 2, 3, 4 or 5
- x and y mutually independently denote an integer≦350
and - z=0 to 200.
- In this connection (I) in copolymer a1) the comonomer units which represent the components 1) and 2) have in each case no internal molecular differences and/or (II) the copolymer a1) represents a polymeric mixture of the components 1) and 2), in which case the comonomer units have internal molecular differences with respect to the radicals R1 and/or R2 and/or R3 and/or R4 and/or R5 and/or R6 and/or R7 and/or m and/or n and/or x and/or y and/or z, and the differences discussed relate in particular to the composition and length of the side chains.
- With regard to the copolymer the disclosure of WO 2006/133933 A2 is a substantial integral of the present disclosure.
- In particular, the present invention comprises a formulation wherein the copolymer a1) contains the comonomer component 1) in proportions of 30 to 99 mol. % and the comonomer component 2) in proportions of 70 to 1 mol. %. A copolymer a1) which contains the comonomer component 1) in proportions of 40 to 90 mol. % and the comonomer component 2) in proportions of 60 to 10 mol. % has been found particularly advantageous in this connection.
- The comonomer component 1) can preferably be an acrylic acid or a salt thereof and the comonomer component 2) in the case where p=0 or 1 a modification which contains a vinyl or allyl group and as the residue R1 a polyether.
- Further, in the context of the present invention, it can be regarded as advantageous if the comonomer component 1) derives from the group acrylic acid, methacrylic acid, crotonic acid, isocrotonic acid, allylsulphonic acid, vinylsulphonic acid and suitable salts thereof and alkyl or hydroxyalkyl esters thereof.
- In addition, the copolymer a1) can have additional structural groups in copolymerized form, which is also taken into account by the present invention. In this case, the additional structural groups may be styrenes, acrylamides and/or hydrophobic compounds, ester structural units, polypropylene oxide and polypropylene oxide/polyethylene oxide units being particularly preferred. The copolymer a1) should contain the said additional structural groups in proportions up to 5 mol. %, preferably from 0.05 to 3.0 mol. % and in particular from 0.1 to 1.0 mol. %.
- In addition, it is advantageous if the formula (I) stands for a polyether containing allyl or vinyl groups.
- With regard to the carboxylate ester modifications a2) and the possible forms thereof, reference is in particular made to EP 0 753 488 B1, the content thereof with regard to the dispersants described in that document being an integral part of the present disclosure.
- Concerning the polycarboxylate ester a2) as preferred comb polymer, the present invention specifies that this ester a2) is a polymer which can be prepared by polymerization of a monomer mixture (I) containing, as the main component, a representative of the carboxylic acid monomer type. An important aspect of component a2) according to the present invention has to be seen in the anti-foaming and/or defoaming and/or surface active properties of such polycarboxylate ester types. This is why the formulation according to the present invention also comprises a combination of an antifoaming/surface active agent with dispersing properties as component a) and the polycondensate component b). In a more preferred embodiment the monomer mixture (I) contains an (alkoxy)polyalkylene glycol mono(meth)acrylate monomer (a) of the general formula (II)
- in which R1 represents a hydrogen atom or a CH3 group, R2O represents one representative or a mixture of at least two oxyalkylene groups having 2 to 4 carbon atoms, R3 represents a hydrogen atom or an alkyl group having 1 to 5 carbon atoms and m represents a number between 1 and 250 and represents the average number of moles of the oxyalkylene group added,
additionally, as monomer (b), a (meth)acrylic acid of the general formula (III), - in which R4 represents a hydrogen atom or a CH3 group and M1 represents a hydrogen atom, a monovalent metal atom, a divalent metal atom, an ammonium group or an organic amine group, and optionally a monomer (c) which is copolymerized with the monomers (a) and (b). The monomer (a) can be present in an amount of from 5 to 98 wt. %, the monomer (b) in a proportion of from 2 to 95 wt. % and the monomer (c) in a proportion up to 50 wt. % in the monomer mixture (I), wherein the respective proportions of the monomers (a), (b) and (c) add up to 100 wt. %.
- As typical representatives of the monomer (a), hydroxyethyl(meth)acrylate, hydroxypropyl(meth)acrylate, polyethylene glycol mono(meth)acrylate, polypropylene glycol mono(meth)acrylate, polybutylene glycol mono(meth)acrylate, polyethylene glycol polypropylene glycol mono(meth)acrylate, polyethylene glycol polybutylene glycol mono(meth)acrylate, polypropylene glycol polybutylene glycol mono(meth)acrylate, polyethylene glycol polypropylene glycol polybutylene glycol mono(meth)acrylate, methoxypolyethylene glycol mono(meth)acrylate, methoxypolypropylene glycol mono(meth)acrylate, methoxypolybutylene glycol mono(meth)acrylate, methoxypolyethylene glycol polypropylene glycol mono(meth)acrylate, methoxypolyethylene glycol polybutylene glycol mono(meth)acrylate, methoxypolypropylene glycol polybutylene glycol mono(meth)acrylate, methoxypolyethylene glycol polypropylene glycol polybutylene glycol mono(meth)acrylate, ethoxypolyethylene glycol mono(meth)acrylate, ethoxypolypropylene glycol mono(meth)acrylate, ethoxypolybutylene glycol mono(meth)acrylate, ethoxypolyethylene glycol polypropylene glycol mono(meth)acrylate, ethoxypolyethylene glycol polybutylene glycol mono(meth)acrylate, ethoxypolypropylene glycol polybutylene glycol mono(meth)acrylate, ethoxypolyethylene glycol polypropylene glycol polybutylene glycol mono(meth)acrylate or mixtures thereof are possible.
- For the monomer (b), representatives of the group consisting of acrylic acid, methacrylic acid, monovalent metal salts, divalent metal salts, ammonium salts and organic amine salts thereof and mixtures of at least two of the said representatives are to be regarded as preferred.
- As regards the monomer (c), the formulation according to the invention should contain at least one representative of the esters of an aliphatic alcohol with 1 to 20 carbon atoms with an unsaturated carboxylic acid. As the unsaturated carboxylic acid, in particular maleic acid, fumaric acid, citraconic acid (meth)acrylic acid or monovalent metal salts, divalent metal salts, ammonium salts or organic amine salts thereof are especially suitable. Monoesters or diesters of unsaturated dicarboxylic acids such as maleic acid, fumaric acid or citraconic acid with aliphatic C1-C20 alcohols, C2-C4 glycols or with (alkoxy)polyalkylene glycols are preferred representatives of monomer (c) according to the present invention.
- 1.2 Copolymer a2:
- In the context of the present invention, the component a2) can be a copolymer which is made up of at least one of the following monomers:
- A) an ethylenically unsaturated monomer, containing a hydrolysable residue
- B) an ethylenically unsaturated monomer with at least one C2-C4 oxyalkylene side group with a chain length from 1 to 30 units;
- C) an ethylenically unsaturated monomer with at least one C2-C4 oxyalkylene side group with a chain length from 31 to 350 units.
- In a preferred embodiment of the present invention components B) and C) are simultaneously represented in the copolymer a2) of the claimed formulation.
- In this copolymer modification, built up of at least one of the monomers A), B) and C), according to the invention the ethylenically unsaturated monomer of the component A) can be at least one anhydride or imide and/or at least one maleic anhydride or maleimide. The ethylenically unsaturated monomer of the component A) can however also include an acrylate ester with an ester functionality which contains the hydrolysable residue. In this case, it should be regarded as preferred if the ester functionality is at least one hydroxypropyl or hydroxyethyl radical.
- In a further embodiment the copolymer a2) can however comprise more than one ethylenically unsaturated monomer with a hydrolysable radical. Here it is in particular recommended that the ethylenically unsaturated monomer of the component A) as a residue has at least more than one representative of the ethylenically unsaturated monomers, at least one representative of a hydrolysable radical or a mixture of both. In this connection, the hydrolysable radical should have at least one C2-C20 alcohol functionality. The present invention also includes the possibility that the hydrolysable residue is at least one C1-C20 alkyl ester, one C1-C20 aminoalkyl ester, one C2-C20 alcohol, one C2-C20 amino alcohol or one amide.
- The present invention further comprises that at least one ethylenically unsaturated monomer of the component B) or C) has a C2-C8 alkyl ether group. In this case, the ethylenically unsaturated monomer can have a vinyl, allyl or (methyl)allyl ether residue or else be derived from an unsaturated C2-C8 alcohol. In the last-named case of the unsaturated C2-C8 alcohol, at least vinyl alcohol, (meth)allyl alcohol, isoprenol or methylbutenol are especially preferred possibilities as representatives.
- The ethylenically unsaturated monomer side groups of the component B) or C) can however also contain at least one C4 oxyalkylene unit.
- Overall, in connection with the modifications just described, concerning the comb polymer a2) it can be stated that at least one ethylenically unsaturated monomer of the components B) or C) can have a C2-C8 carboxylate ester, which in particular is hydrolysable. Further, the present invention includes a modification wherein the oxyalkyl side groups have at least one ethylene oxide, one propylene oxide, one polyethylene oxide, one polypropylene oxide or mixtures thereof.
- Finally, the copolymer a2) in the component C) can have at least one nonionic (“uncharged”) and/or one non-hydrolysable monomer residue or mixtures thereof.
- 1.3 Copolymer a3:
- In addition to the two modifications just described in detail with regard to the component a), namely its form as polycarboxylate ethers and polycarboxylate esters, the present invention also includes a third modification of the comb polymer a), which then is a nonionic (uncharged) copolymer a3). Here, representatives of the general formula (IV) are preferred,
- wherein Q stands for an ethylenically unsaturated monomer with at least one hydrolysable residue, G means O, C(O)—O or O—(CH2)p-O with p=2 to 8, wherein mixtures of the modifications of G in one polymer are possible; R1 and R2 mutually independently mean at least one C2-C8 alkyl; R3 comprises (CH2)c, where c is a whole number between 2 and 5 and where mixtures of the representatives of R3 in the same polymer molecule are possible; R5 means at least one representative selected from the series H, a linear or branched, saturated or unsaturated C1-C20 aliphatic hydrocarbon residue, a C5-C8 cycloaliphatic hydrocarbon residue or a substituted or unsubstituted C6-C14 aryl residue; m=1 to 30, n=31 to 350, w=1 to 40, y=0 to 1 and z=0 to 1, where the sum (y+z)>0.
- However, the nonionic copolymer a3) can alternatively also be a representative of the general formula (V),
- wherein X stands for a hydrolysable residue and R for H or CH3, and G, p, R1, R2, R3, R5, m, n, w, y, z and (y+z) have the meanings stated under the formula (IV).
- In the case where the structure of the non-ionic copolymer a3) corresponds to the formula (V), in a preferred embodiment the hydrolysable residue can be at least one representative of the series alkyl ester, aminoalkyl ester, hydroxyalkyl ester, aminohydroxyalkyl ester or amide.
- As a third alternative as regards the nonionic copolymer a3), the present invention specifies at least one representative of the general formula (VI)
- wherein R4 means at least one C1-C20 alkyl or C2-C20 hydroxyalkyl radical and the radicals G, p, R, R1, R2, R3, c, R4, R5 and m, n, w, y, z and (y+z) have the meanings stated under the formulae (IV) and (V).
- It is to be regarded as preferred option that in this formula (VI), p=4, R4═C2H4OH or C3H6OH, each of the radicals R5 represents H, m=5-30, n=31-250, w=1.5-30,y=0 to 1,z=0 to 1 and (y+z)>0.
- Further it is to be regarded as preferred embodiment that in the said formulae (IV), (V) and (VI), the molar ratio of w to the sum (y+z) is 1:1 to 20:1 and preferably 2:1 to 12:1.
- The representative of the third modification of the copolymer a3) corresponding to formula (VI) should in particular be a nonionic polyether-polyester copolymer.
- The terms “nonionic” and “uncharged” are to be understood as synonyms in this context.
- Irrespective of the component a) and its preferred representatives a1), a2) and/or a3), respectively contained in the formulation according to the invention, the present invention specifies that the formulation contain the component a) in proportions from 5 to 95% by weight, preferably of 10 to 60% by weight and particularly preferably of 15 to 40% by weight, based in each case on the total formulation.
- Sulphonic acid group containing s-triazines or naphthalene-formaldehyde condensates are broadly disclosed by prior art documents and frequently used as water reducing agents or plasticizers for cement based systems such as concrete.
- β-naphthalene-sulphonate-formaldehyde condensates (“BNS”), also known as naphthalene-formaldehyde sulphonates (“NFS”) disperse cement particles by an electrostatic repulsion that results from adsorption processes.
- BNS or NFS is suitable for making cement particles with high dispersion, low foaming and high range water reducing and thereof it is possible to save the hydraulic binder such as cements or calcium sulphite based binders to improve the cement mobility and workability. BNS is a high range admixture for concrete, cast-in-place, prefabricating, pump and curing and BNS has a good adaptability to cements and other hydraulic binders and is not corrosive to reinforcing bar and non poisonous and pollution-free. Therefore it has been broadly applied to the construction industry such as highways, bridges, tunnels, industrial buildings, prestressing force components and high range concretes.
- Usually, such condensates suitable as plasticizer or dispersants are prepared by the reaction of aromatic sulphonic acids like naphthalene sulphonic acid with formaldehyde under ambient pressure and under temperatures up to 100° C.
- The preparation and use of BNS is well known state of the art and disclosed for example in EP 0 214 412 A1 and DE-PS 2 007 603.
- The effect and properties of BNS can be modified by changing the molar ratio between formaldehyde and the naphthalene component that usually is from 0.7 up to 3.5. The ratio between formaldehyde and the sulphonated naphthalene component preferably is from 0.8 to 3.5 to 1.
- BNS condensates are added to the hydraulic binder containing composition in amounts from 0.01 up to 6.0 wt. %.
- Melamine-sulphonate-formaldehyde-condensates (“MFS”) are broadly used as flow improving agents in the processing of hydraulic binder containing compositions such as dry mortar mixtures, pourable mortars and other cement bonded construction materials.
- Melamine is used in this connection as representative of the s-triazine which is why these improving agents are known as MFS resins. They cause as well as the already mentioned BNS representatives a strong liquefying effect of the construction chemicals mixture without any undesired side effects occurring in the processing or in the functional properties of the hardened building material.
- It is well known that commercially available flow improving agents based on melamine-formaldehyde-sulphite such as products of the Melment series of BASF Construction Polymers GmbH, Germany, cause an excellent liquefying effect even of low dosages of about 0.3 to 1.2 wt. %, relative to the weight of the hydraulic binder such as cement.
- The liquefying effect of MFS products is achieved without lowering the surface tension of the water and binder system which usually is the case for the example with BNS products or flow improving agents with a surfactant-like polymers structure. The advantage of MFS resins is presumed to be due to the fact that no air avoids are introduced in to mortar during remixing process and the mortar density and strengths are not adversely effected after hardening.
- In addition MFS resins provide the fresh mortar mixture with a good cohesive strength so that even when the flow properties are extreme separation phenomena within the construction composition do not occur. This phenomenon, also called as “segregation”, is feared especially in the production of self-flowing smoothing compositions which especially is the case with self-leveling screeds since its leads to a non-uniform layer structure of the screed due to floating of the fine material and sedimentation of the coarse grain.
- As it is for the BNS technology also for MFS there is a broad prior art. In this connection as representative documents are mentioned DE 196 09 614 A1, DE 44 11 797 A1, EP 0 059 353 A1 and DE 195 38 821 A1:
- DE 196 09 614 A1 discloses a water soluble polycondensation product based on an amino-s-triazine and its use as plasticizer in aqueous binder containing suspensions based on cement, lime and gypsum. These polycondensates are capable in two condensation steps whereby in a pre-condensation step the amino-s-triazine, the formaldehyde component and the sulphite are condensated at a molar ratio of 1 to 0.5:5.0 to 0.1:1.5. Melamine is a preferred representative of amino-s-triazines. Further suitable representatives are amino plast former selected from the group urea, thiourea, dicyandiamide or guanidine and guanidine salts.
- According to DE 44 11 797 A1 sulfanilic acid containing condensation products based on amino-s-triazines that show at least two amino groups are prepared by using formaldehyde. The sulfanilic acid is used in amounts of from 1.0 to 1.6 mol per mol amino-s-triazine and neutralized in aqueous solution with an alkaline metal hydroxide or in earth alkaline metal hydroxide. In an additional step the formaldehyde is added in amounts of from 3.0 to 4.0 mol per mol amino-s-triazine at a pH value between 5.0 to 7.0 and at temperatures between 50 and 90° C. The final viscosity of the solution shall be between 10 and 60 cSt at 80° C.
- According to EP 0 059 353 A1 highly concentrated and low viscose aqueous solutions of melamine/aldehyde resins are capable by reacting melamine and an aldehyde in an alkaline medium in a first step with a component selected from the group comprising alkali sulphate, earth alkali sulphate or (earth) alkali sulphonate or other suitable amino compounds to a pre-condensate. This mixture in an additional process step is reacted with another amino compound such as amino acids or amino carbonic acids and finally the resin solution is brought to an alkaline pH value.
- DE 195 38 821 A1 discloses a condensate based on an amino-s-triazine with at least two amino groups and formaldehyde and a high content of sulphonic acid groups and a low content of formate. Such products can be prepared according to this document by reacting the amino-s-triazine, formaldehyde and a sulphite at a molar ratio of 1:3.0:6.0:1.51:2.0 in an aqueous solution and at a temperature between 60 and 90° C. and a pH value between 9.0 and 13.0 until the sulphite is no longer present. In an additional step the condensation process is conducted at a pH value between 3.0 and 6.5 and at temperatures between 60 and 80° C. until the condensation product at 80° C. shows a viscosity between 5 and 50 mm2/s. Finally, the condensation product is to be brought to a pH value between 7.5 and 12.0 or treated thermally by a pH≧10.0 and a temperature between 60 and 100° C.
- According to the present invention the BNS and/or MFS dispersant is used in amounts of from 0.01 to 10 wt. % and preferably 0.1 to 5 wt. %, related to the hydraulic binder component. The molar ratio of the sulphonic group and related to the melamine component is of from 1.0 to 2.0 and the molar ratio of the formaldehyde related to the melamine component is from 2.5 to 5.0. Preferably the molar ratio melamine to sulphonic acid to formaldehyde is 1:1.1:1.5:3.3:3.6.
- Concerning the BNS component the molar ratio of formaldehyde to naphthalene sulphonic acid is from 1.3 to 1:3 to 1.
- As already discussed as state of the art admixtures in the form of dispersants are added to aqueous slurries or pulverulent inorganic or organic substances, such as clays, silicate powder, chalk, carbon black, crushed rock and hydraulic binders, for improving their processability, i.e. kneadability, spreadability, sprayability, pumpability or flowability. Such admixtures are capable of preventing the formation of solid agglomerates and of dispersing the particles already present and those newly formed by hydration and in this way improving the processability. This effect is utilized in particular in a targeted manner in the preparation of construction material mixtures which contain hydraulic binders, such as cement, lime, gypsum, hemihydrate or anhydrite.
- In order to convert these construction material mixtures based on said binders, into a ready-to-use, processable form, as a rule substantially more mixing water is required than would be necessary for the subsequent hydration or hardening process. The proportion of voids which is formed in the concrete body by the excess, subsequently evaporating water leads to significantly poorer mechanical strengths and resistances.
- In order to reduce this excess proportion of water at a predetermined processing consistency and/or to improve the processability at a predetermined water/binder ratio, admixtures are used which are generally referred to as water-reducing agents or plasticizers. In practice, in particular polycondensates and copolymers are used as such agents.
- WO 2006/042709 describes polycondensates based on an aromatic or heteroaromatic compound (A) having 5 to 10 C atoms or heteroatoms, having at least one oxyethylene or oxypropylene radical, and an aldehyde (C) selected from the group consisting of formaldehyde, glyoxylic acid and benzaldehyde or mixtures thereof, which result in an improved plasticizing effect of inorganic binder suspensions compared with the conventionally used polycondensates and maintain this effect over a longer period (“slump retention”). In a particular embodiment, these may also be phosphated polycondensates. The phosphated monomers used are, however, relatively expensive since they have to be separately prepared and purified.
- Alternatively, there has been developed an economical dispersant, based on a phosphated polycondensate, for hydraulic binders, which dispersant is particularly suitable as a plasticizer/water-reducing agent for concrete and can be prepared in a simple manner and at low costs (non-disclosed prior art filed provisional as EP 081659155.3 in August 2008).
- This object is achieved by a polycondensate containing
-
- (I) at least one structural unit having an aromatic or heteroaromatic and a polyether side chain and
- (II) at least one phosphated structural unit having an aromatic or heteroaromatic and
- (III) at least one structural unit having an aromatic or heteroaromatic,
- structural unit (II) and structural unit (III) differing exclusively in that the OP(OH)2 group of the structural unit (II) is replaced by H in structural unit (III), and structural unit (III) is not the same as structural unit (I).
- The structural units (I), (II) and (III) of component b) of the claimed formulation can be described in more detail by the following general formulae
- where
- A are identical or different and are represented by a substituted or unsubstituted aromatic or heteroaromatic compound having 5 to 10 C atoms
where - B are identical or different and are represented by N, NH or O
where - n=2, if B=N and n=1, if B=NH or O
where - R1 and R2, independently of one another, are identical or different and are represented by a branched or straight-chain C1- to C10-alkyl radical, C5- to C8-cycloalkyl radical, aryl radical, heteroaryl radical or H
where - a are identical or different and are represented by an integer from 1 to 300
where - X are identical or different and are represented by a branched or straight-chain C1- to C10-alkyl radical, C5- to C8-cycloalkyl radical, aryl radical, heteroaryl radical or H,
- for (VIII) and (IX) in each case:
where - D are identical or different and are represented by a substituted or unsubstituted heteroaromatic compound having 5 to 10 C atoms
where - E are identical or different and are represented by N, NH or O
where - m=2if E=N and m=1 if E=NH or O
where - R3 and R4, independently of one another, are identical or different and are represented by a branched or straight-chain C1- to C10-alkyl radical, C5- to C8-cycloalkyl radical, aryl radical, heteroaryl radical or H
where - b are identical or different and are represented by an integer from 0 to 300,
where - M is independently of one another an alkaline metal ion, alkaline earth metal ion, ammonium ion, organic ammonium ion and/or H,
- c is 1 or in the case of alkaline earth metal ions ½.
- In a preferred embodiment, the polycondensate contains a further structural unit (X) which is represented by the following formula
- where
- Y, independently of one another, are identical or different and are represented by (VII), (VIII), (IX) or further constituents of the polycondensate
where - R5 are identical or different and are represented by H, CH3, COOMc or a substituted or unsubstituted aromatic or heteroaromatic compound having 5 to 10 C atoms
where - R6 are identical or different and are represented by H, CH3, COOMc or a substituted or unsubstituted aromatic or heteroaromatic compound having 5 to 10 C atoms.
- Here, R5 and R6 in structural unit (X), independently of one another, are preferably represented by H, COOMc and/or methyl.
- The molar ratio of the structural units (VII), (VII), (IX) and (X) of the phosphated polycondensate according to the invention can be varied within wide ranges. This has proved to be expedient if the molar ratio of the structural units [(VII)+(VII)+(IX)]:(X) is 1:0.8 to 3, preferably 1:0.9 to 2 and particularly preferably 1:0.95 to 1.2.
- The molar ratio of the structural units (VII):[(VIII)+(IX)] in component b) is usually 1:15 to 15:1, preferably 1:10 to 10:1 and more preferably 1:5 to 3:1.
- In a preferred embodiment, the molar ratio of the structural units (VIII):(IX) is adjusted to 1:0.005 to 1:10, preferably 1:0.01 to 1:1, in particular 1:0.01 to 1:0.2 and more preferably 1:0.01 to 1:0.1.
- The groups A and D in the structural units (VII), (VIII) and (IX) of the polycondensate are generally represented by phenyl, 2-hydroxyphenyl, 3-hydroxyphenyl, 4-hydroxyphenyl, 2-methoxyphenyl, 3-methoxyphenyl, 4-methoxyphenyl, naphthyl, 2-hydroxynaphthyl, 4-hydroxynaphthyl, 2-methoxynaphthyl, 4-methoxynaphthyl, preferably phenyl, it being possible for A and D to be chosen independently of one another and also in each case to consist of a mixture of said compounds. The groups B and E, independently of one another, are preferably represented by O.
- The radicals R1, R2, R3 and R4 can be chosen independently of one another and are preferably represented by H, methyl, ethyl or phenyl, particularly preferably by H or methyl and especially preferably by H.
- A in structural unit (VII) is preferably represented by an integer from 5 to 280, in particular 10 to 160 and particularly preferably 12 to 120 and b in structural units (VIII) and (IX) by an integer from 0 to 10, preferably 1 to 7 and particularly preferably 1 to 5. The respective radicals, the length of which is defined by a and b, respectively, may consist here of uniform building blocks, but a mixture of different building blocks may also be expedient. Furthermore, the radicals of the structural units (VII) or (VIII) and (IX), independently of one another, may each have the same chain length, a and b each being represented by a number. As a rule, however, it will be expedient if mixtures having different chain lengths are present in each case so that the radicals of the structural units in the polycondensate have different numerical values for a and independently for b.
- Frequently, the phosphated polycondensate according to the present invention has a weight average molecular weight of 4000 g/mol to 150 000 g/mol, preferably 10 000 to 100 000 g/mol and particularly preferably 20 000 to 75 000 g/mol.
- As a rule, the phosphated polycondensate according to the invention is present in the claimed formulation as aqueous solution which contains 2 to 90% by weight of water and 98 to 10% by weight of dissolved dry matter, preferably 40 to 80% by weight of water and 60 to 20% by weight of dissolved dry matter, and more preferably 45 to 75% by weight of water and 55 to 25% by weight of dissolved dry matter. The dry matter then substantially comprises the anhydrous phosphated polycondensate, where further components, such as antifoams and other auxiliaries, can advantageously also be present.
- In a further embodiment the polycondensate b) is present in the formulation in proportions of 5 to 100% by weight, preferably of 10 to 60% by weight and particularly preferably of 15 to 40% by weight, based in each case on the total formulation.
- In a particular embodiment, the invention furthermore envisages a sodium, potassium, ammonium and/or calcium salt and preferably a sodium and calcium salt, of the phosphated polycondensate.
- The present invention also relates to a process for the preparation of a phosphated polycondensate, it being regarded as essential that the polycondensation and the phosphation be carried out in a reaction mixture. This is to be understood as meaning that the phosphated component formed in the reaction solution needs neither be purified nor isolated. The phosphation can be carried out before, during or after the polycondensation. It is to be regarded as being preferred here to carry out both the phosphation and the polycondensation in the same reaction vessel.
- In a preferred embodiment, the reaction mixture with regard to the polycondensate component b) contains at least
- (Ia) a monomer having a polyether side chain and an aromatic or heteroaromatic,
- (IIIa) a monomer having an aromatic or heteroaromatic unit, (IIIa) being partially phosphated during the reaction and forming the monomer (IIa) and/or, in the polycondensate, the structural unit (IIa),
- (IVa) a monomer having an aldehyde group and a phosphating agent,
- structural unit (IIIa) not being the same as structural unit (Ia).
- The monomers (Ia), (IIa), (IIIa) and (IVa) and, in the polycondensate, the structural unit (IIa) are preferably represented by the following general formulae:
-
- where
- A are identical or different and are represented by a substituted or unsubstituted aromatic or heteroaromatic compound having 5 to 10 C atoms
where - B are identical or different and are represented by N, NH or O
where - n=2 if B=N and n=1 if B=NH or O
where - R1 and R2, independently of one another, are identical or different and are represented by a branched or straight-chain C1- to C10-alkyl radical, C5- to C8-cycloalkyl radical, aryl radical, heteroaryl radical or H
where - a are identical or different and are represented by an integer from 1 to 300
where - X are identical or different and are represented by a branched or straight-chain C1- to C10-alkyl radical, C5- to C8-cycloalkyl radical, aryl radical, heteroaryl radical or H;
-
-
- for formulae (VIIIa) and (IXa) in each case:
where - D are identical or different and are represented by a substituted or unsubstituted heteroaromatic compound having 5 to 10 C atoms
where - E are identical or different and are represented by N, NH or O
where - m=2 if E=N and m=1 if E=NH or O
where - R3 and R4, independently of one another, are identical or different and are represented by a branched or straight-chain C1- to C10-alkyl radical, C5- to C8-cycloalkyl radical, aryl radical, heteroaryl radical or H
where - b are identical or different and are represented by an integer from 0 to 300;
-
- where
- R7 are identical or different and are represented by H, CH3, COOH and/or a substituted or unsubstituted aromatic or heteroaromatic compound having 5 to 10 C atoms
where - R8 are identical or different and are represented by H, CH3, COOH and/or a substituted or unsubstituted aromatic or heteroaromatic compound having 5 to 10 C atoms.
- The present invention provides different variants of the reaction procedure. One possibility consists in first reacting the monomer (IIIa) with a phosphating agent and subjecting the monomer (IIa) thus obtained to polycondensation with the monomers (Ia), (IIIa) and (IVa). The monomer (IIIa) may originate here from an incomplete reaction during the phosphation reaction or can be deliberately added to the reaction mixture after the phosphation reaction.
- However, it is also possible to subject the monomers (Ia), (IIIa) and (IVa) to a polycondensation and then to react the polycondensate obtained with a phosphating agent. In a further embodiment, the monomers (Ia), (IIIa) and (IVa) and the phosphating agent are reacted simultaneously.
- In particular, polyphosphoric acid and/or phosphorous pentoxide have proved suitable here as phosphating agents.
- As a rule, the polycondensation is carried out in the presence of an acidic catalyst, this preferably being sulphuric acid, methanesulphonic acid, para-toluenesulphonic acid or mixtures thereof.
- The polycondensation and the phosphation are advantageously carried out at a temperature between 20 and 140° C. and a pressure between 1 and 10 bar. In particular, a temperature range between 80 and 110° C. has proved to be expedient. The duration of the reaction may be between 0.1 and 24 hours, depending on temperature, the chemical nature of the monomers used and the desired degree of crosslinking. Once the desired degree of crosslinking has been reached, which can also be determined, for example, by measurement of the viscosity of the reaction mixture, the reaction mixture is cooled.
- According to a particular embodiment, the reaction mixture is subjected to a thermal aftertreatment at a pH between 8 and 13 and a temperature between 60 and 130° C. after the end of the condensation and phosphation reaction. As a result of the thermal aftertreatment, which advantageously lasts for between 5 minutes and 5 hours, it is possible substantially to reduce the aldehyde content, in particular the formaldehyde content, in the reaction solution.
- In a further particular embodiment, the present invention envisages subjecting the reaction mixture to a vacuum aftertreatment at pressures between 10 and 900 mbar after the end of the condensation and phosphation reaction, for reducing the aldehyde content. Furthermore, however, other methods known to the person skilled in the art for reducing the formaldehyde content may also be used. An example is the addition of small amounts of sodium bisulphite, ethylene urea and/or polyethylenimine.
- The phosphated polycondensates obtained by these processes can be used directly as component b). In order to obtain a better shelf life and better product properties, it is advantageous to treat the reaction solutions with basic compounds. It is therefore to be regarded as being preferred to react the reaction mixture after the end of the reaction with a basic sodium, potassium, ammonium or calcium compound. Sodium hydroxide, potassium hydroxide, ammonium hydroxide or calcium hydroxide has proved to be particularly expedient here, it being regarded as being preferred to neutralize the reaction mixture. However, other alkali metal and alkaline earth metal salts and salts of organic amine are suitable as salts of the phosphated polycondensates.
- Furthermore, however, the present invention also provides the preparation of mixed salts of the phosphated polycondensates. These can expediently be prepared by reacting the polycondensates with at least two basic compounds.
- Thus, by a targeted choice of suitable alkali metal and/or alkaline earth metal hydroxides, it is possible by neutralization to prepare salts of the polycondensates according to the invention, with which the duration of the processability of aqueous suspensions of inorganic binders and in particular of concrete can be influenced. While a reduction in the processability over time is observable in the case of the sodium salt, a complete reversal of this behavior takes place in the case of the calcium salt of the identical polymer, a smaller water reduction (smaller slump) occurring at the beginning and increasing with time. As a result of this, sodium salts of the phosphated polycondensates lead over time to a decrease in the processability of the binder-containing material, such as, for example, concrete, mortar or gypsum slurries, whereas the corresponding calcium salts lead with time to improved processability. By suitable choice of the amount of sodium and calcium salts of the phosphated polycondensates used, the development of the processability of binder-containing materials can thus be controlled as a function of time. Expediently, the corresponding phosphated polycondensates, which consist of sodium and calcium salts, are prepared by reaction with a mixture of basic calcium and sodium compounds, in particular calcium hydroxide and sodium hydroxide.
- According to the present invention, the catalyst used can also be separated off. This can expediently be effected via the salt formed during the neutralization. If sulphuric acid is used as a catalyst and the reaction solution is treated with calcium hydroxide, the calcium sulphate formed can be separated off, for example, in a simple manner by filtration.
- Furthermore, by adjusting the pH of the reaction solution to 1.0 to 4.0, in particular 1.5 to 2.0, the phosphated polycondensate can be separated from the aqueous salt solution by phase separation and can be isolated. The phosphated polycondensate can then be taken up in the desired amount of water.
- However, other methods known to the person skilled in the art, such as dialysis, ultrafiltration or the use of an ion exchanger, are also suitable for separating off the catalyst.
- Surprisingly, with a phosphated polycondensate according to the invention as component b) of the formulation an improved efficiency was found in comparison with the polycondensates known in the prior art. As additional favorable effect a significantly decreased retardation of the setting and hardening of the various construction compositions compared to other dispersants is to be observed, independently from the dosage of component b). This effect of the polycondensate component b) as well as an expedient influence on the pore structure surprisingly can be observed also with the formulation according to the present invention.
- Additionally, it has proved particularly advantageous that [the phosphated polycondensates according to the invention] can be prepared by a very economical process, no further purification of intermediates being required. In particular, no wastes which have to be disposed of form in the process according to the invention. Thus, the claimed process also constitutes further progress compared with the prior art from environmental points of view. The reaction mixture obtained can be put directly to the intended formulation optionally after treatment with basic compounds.
- In a specific embodiment the claimed formulation contains additionally to the components a) and b) at least one antifoaming agent c) and/or a component d) having a surface-active effect, the components c) and d) being structurally different from one another.
- The antifoaming agent c) is preferably selected from the group consisting of a mineral oil, a vegetable oil, a silicon oil, a silicon containing emulsion, a fatty acid, a fatty acid ester, an organic modified polysiloxane, a borate ester, an alkoxylate, a polyoxialkylene copolymer, ethylene oxide (EO)-propylene oxide (PO) block polymer, acetylenic diols having defoaming properties and a phosphoric ester having the formula P(O) (O—R8)3-x(O—R9)x wherein P represents phosphorus, O represents oxygen and R8 and R9 are independently a C2-C20 alkyl or an aryl group and x=0, 1, 2, whereby an alkyl group with C2-C8 is preferred.
- Preferably said antifoaming agent c) comprises tri-alkylphosphate and more preferably triiso-butylphosphate, a polyoxypropylen copolymer and a glycerol/alcohol acetate.
- The invention additionally comprises an admixture wherein said antifoaming agent c) comprises a mixtures of a tri-alkylphosphate and a polyoxypropylene copolymer.
- The second optional component of the formulation, namely the surfactant, is preferably selected from the group consisting of a ethylene oxide/propylene oxide (EO/PO) block copolymer, a styrene/maleic acid copolymer, a fatty alcohol alkoxylate, an alcohol ethoxylate R10-(EO)—H with R10 being an aliphatic hydrocarbon group having from 1 to 25 carbon atoms, acetylenic diols, monoalkylpolyalkylenes, ethoxylated nonylphenols, alkylsulfates, alkylethersulfats, alkylethersulfonates, alkyl ether carboxylates.
- More preferably surfactant component d) comprises an alcohol having a polyalkylene group consisting of a carbon chain length of 2 to 20 carbon atoms, with a specific carbon chain length of C3-C12.
- Advantageously the formulation according to the invention comprises an aqueous composition that contains the antifoaming agent component c) in free form or attached to the dispersing components a), and/or b). If the antifoaming agent is attached to the dispersing components it can be physically or chemically attached, and the chemically attached in this case in polymerized and/or grafted form being preferred. When chemically attached, the antifoaming agent c) also can be considered as a third co-monomer of the copolymeric dispersing components a1), a2), a3). In its free form the antifoaming agent c) is a blend component of the formulation. Thus, antifoaming agent component c) is either physically and/or chemically attached to the dispersing components a1), a2) and/or a3) and/or it is a free form component and therefore constituent of a blend.
- In a further embodiment the antifoaming component c) is present in amounts of 0.01 to 10% by weight and/or the surface-active component d) is present in amounts of 0.01 to 10% by weight, based in each case on the total weight of the formulation. According to a preferred embodiment the antifoaming formulation according to any of Claims 52 to 61, characterized in that the antifoam c) and/or the surface-active component d), independently of one another, are present in each case in an amount of 0.01 to 5% by weight, based in each case on the total weight of the formulation. The present invention additionally comprises an embodiment whereby the formulation in addition to the components a) and b) and optionally c) and/or d), contains at least one further compound e) selected from the group consisting of a polymer having a low charge, a neutral polymer or polyvinyl alcohol. This component e) and particularly its specific role in systems containing calcium sulfate as hydraulic binder has been teached in the unpublished provisional European Patent application EP 08171022.0. The component e) plays a major role in gypsum composition with certain clay contents.
- In the use of clay-containing forms of gypsum, and particularly natural gypsum, it can be observed that considerable quantities of the dispersant (fluidizing agent) used are absorbed or adsorbed by the clay mineral, as a result of which they are no longer available for the fluidization of the gypsum hemihydrate in the gypsum mixture.
- To solve this problem, attempts were made to use so-called sacrificial substances, which in competition with the dispersant bind more strongly to the surface of the clay particles and in this way either mask these so that they are no longer accessible to the dispersant, or largely flocculate the clay particles.
- According to the mentioned European application there has been provided a formulation based on a branched comb polymer with ethylene oxide (EO) units in the side-chains for the dispersion of clay-containing gypsum mixtures. These formulations are capable of masking clay minerals such as are in particular contained in natural gypsum to a sufficient extent that the surfaces thereof are no longer available for the adsorption of dispersants. They have no adverse effect on the fluidization and consistency of the wet and unhardened gypsum mixture and they are stable to the temperatures used in the drying of the gypsum products, so that no odour problems arise.
- In this connection and with regard to clay-containing gypsum compositions a copolymer component a2) is to prefer that is based on a hydrolysable monomer A having an active binding site for at least one component of the clay-containing gypsum mixture.
- With component e) according to the present invention, the surface of the clay particles can be more effectively coated through the bunching of flexible EO side-chains on a polymer backbone or the clay particles can themselves be better flocculated overall. Because of the lower charge density, the component e) can adsorb mainly on the clay and not on the binder such as gypsum hemihydrate.
- Evidently a not insignificant role in the effects is played by the side-chains of the “sacrificial substance”. These must include EO units; however, the side-chains can also in addition have polyethylene oxide (PO) units. The same applies for the main substance contained in the formulation according to the invention, the comb polymer with dispersant properties; this can contain either EO or PO units or both in its side-chains. Here the mixed modifications can also each be implemented in at least one, that is the same, side-chain.
- Overall, it can be stated that from the chemical point of view the component e) optionally contained in the formulation according to the invention as a sacrificial substance to some extent differs only insignificantly from the dispersants a) commonly used in clay-containing gypsums, since it also consists inter alia of polycarboxylate ethers. The difference consists however in the charge state, since only representatives with low or neutral charge are possible as the sacrificial substance. In other words, the manufacture of gypsum products in particular can also be effected with the aid of dispersants which inter alia consist of copolymer mixtures wherein the low-charge or neutral polymer fractions predominantly mask the clay minerals and thus enable the remaining dispersant content to exert its actual fluidizing agent action.
- The advantageous action of the formulation according to the present invention and mainly based on component e) is displayed in essentially all clay-containing gypsum mixtures. However, the positive action is especially pronounced in gypsum systems which contain at least one representative of the series calcium sulphate, calcium sulphate semihydrate or calcium sulphate hemihydrate, anhydrite and gypsum.
- The clay fraction in the gypsum mixture should preferably be swellable and in particular water-swellable and derive from the series of the smectites, montmorillonites, bentonites, vermiculites, hectorites or from the series of the kaolins, feldspars and micas such as for example illite and mixtures thereof.
- Essentially, care should be taken that the clay contents in the gypsum mixtures do not exceed certain limits. For this reason, the present invention recommends clay contents in the gypsum mixtures of ≦10 wt. %, preferably ≦6 wt. %, preferably ≦4 wt. % and especially preferably between 0.5 and 3 wt. %, each based on the gypsum component.
- For the polymer component e), proportions from 0.01 to 0.40 wt. %, preferably from 0.02 to 0.30 wt. %, preferably from 0.03 to 0.15 wt. % and especially preferably from 0.5 to 0.10 wt. %, each again based on the gypsum component, are recommended.
- In a further embodiment of the invention the formulation contains the component e) in amounts of 1 to 50% by weight, preferably of 5 to 40% by weight and particularly preferably in amounts of 10 to 30% by weight, based in each case on the total weight of the formulation.
- In the context of the present invention, the polymer component e), which reacts with the clay particles in the gypsum mixture, is of particular significance. In the case of a low-charge polymer as component e) this should be branched, the side-chain preferably consisting of a polyether. Polycarboxylate ethers and/or polycarboxylate esters, preferably with EO side-chains and with a carboxylate content up to 83 mol. %, and preferably up to 75 mol. % are to be regarded as especially preferred in this connection.
- As already stated, component a) of the formulation should advantageously include at least one polycarboxylate derivative (ether, ester); in particular if this has a low charge content, it cannot on account of its specific properties adsorb for example onto gypsum to the necessary extent. For this reason, the generally known dispersant action of polycarboxylate ethers and esters in particular does not occur to the necessary extent in this case. Hence the content of the charge-bearing component is important for the dispersant action of such representatives. Since the copolymer components a1), a2) and a3) and, to some extent, depending on its chemical character, also component e) can compete with one another as regards the dispersant action, it is advantageous overall to select the respective contents in the formulation according to the invention such that the copolymer component a) can exhibit its dispersant action to the maximum and the component e) because of its charge properties has as little dispersant action as possible, but instead is maximally adsorbed on the clay particles.
- If a low-charge polymer with a polyether side-chain is used as component e), then this should be made up of at least one monomer selected from the series polyether monoacrylate, polyether monomethacrylate, polyether monoallyl ether, polyether monomaleate, monovinylated polyether or mixtures thereof. In the case of a polyether, this can be an alkylene oxide polymer with a molecular weight from 500 to 10 000, preferably from 750 to 7500 and in particular from 1000 to 5000. As representative alkylene oxide polymers, those based on an ethylene oxide, propylene oxide, butylene oxide or mixtures thereof may be mentioned.
- Low-charge polymers which are built up of at least one monomer selected from the series polypropylene glycol acrylates, polypropylene glycol methacrylates, polyethylene glycol acrylates, polyethylene glycol methacrylates, polypropylene glycol monovinyl ethers, polythylene glycol monovinyl ethers, alkoxy or aryloxypolyethylene glycol acrylates, alkoxy or aryloxypolyethylene glycol methacrylates, alkoxy or aryloxy-polyethylene glycol monovinyl ethers, acrylates, methacrylates and monovinyl ethers of an oxyethylene and oxypropylene block or randomized copolymer, polypropylene glycol allyl ether, polyethylene glycol allyl ether, polyethylene glycol monomaleate, polypropylene glycol monomaleate and any mixtures thereof have been found especially suitable.
- It can be seen as preferred embodiment that the polymer e) having a low charge carries a carboxylic acid group, preferably selected from the series consisting of acrylic acid, methacryl acid, maleic acid, fumaric acid, itaconic acid or anhydrides thereof.
- According to the invention, the low-charge polymer can also bear a carboxylic acid and/or sulphonic acid groups. In this case, the present invention specifies that the carboxylic acid group is preferably at least one representative of the series acrylic acid, methacrylic acid, maleic acid, fumaric acid, itaconic acid or anhydrides thereof. 2-Acrylamido-2-methylpropanesulphonic acid (AMPS), vinylsulphonic acid, allyl ether sulphonic acid, 2-sulphoethylmethacrylic acid, styrenesulphonic acid, methallyl-sulphonic acid, and sodium, potassium and ammonium salts and any mixtures thereof, are preferred representatives of compounds which make sulphonic acid groups available. AMPS and vinylsulphonic acid are to be regarded as especially preferable.
- In the case of neutral polymers as component e), these should be made up of neutral monomer building blocks, which are in particular selected from the series acrylic acid alkyl esters and methacrylic acid alkyl esters and hydroxyalkyl esters thereof with up to 5 carbon atoms. Particularly suitable in this case are hydroxyethyl acrylate and hydroxypropyl acrylate and hydroxyethyl methacrylate and hydroxypropyl methacrylate. Also possible are vinyl acetate, N-vinylpyrrolidone, N-vinylcaprolactam, styrene and methylstyrene.
- In a further embodiment the present invention relates to a formulation that contains as additional further component f) a calcium-silicate-hydrate (C-S-H) containing composition.
- It is well known to a skilled person that admixtures for building material mixtures comprising hydraulic binders typically also contain hardening accelerators which shorten the setting time of the hydraulic binder. According to WO 02/070425, calcium silicate hydrate (C-S-H), in particular present in dispersed (finely or particularly finely dispersed) form, can be used as such a hardening accelerator. However, commercially available C-S-H or corresponding C-S-H dispersions may be regarded only as hardening accelerators which have little effect.
- By the non-published provisional application EP 08163468.5 of September 2008 a composition acting as a plasticizer and moreover showing a good performance as a hardening accelerator has been provided.
- According to the present invention the C-S-H containing composition is prepareble by reaction of a water-soluble calcium containing compound with a water-soluble silicate containing compound, the reaction of the water-soluble calcium containing compound with the water-soluble silicate containing compound being carried out in the presence of an aqueous solution preferably containing a water-soluble copolymer that preferably is a dispersant for hydraulic binders and selected from at least a representative of component a) and/or b).
- In principle, only relatively slightly water-soluble compounds are also suitable in each case as water-soluble calcium compounds and water-soluble silicate compounds, although readily water-soluble compounds (which dissolve completely or virtually completely in water) are preferred in each case. However, it must be ensured that a reactivity sufficient for the reaction is present in an aqueous environment with the corresponding reactant (either water-soluble calcium compound or water-soluble silicate compound). It is probably to be assumed that the reaction takes place in aqueous solution but a water-insoluble inorganic compound (C-S-H) is usually present as a reaction product.
- In the context of the present invention, comb polymers are to be understood as meaning those polymers which have relatively long side chains (having a molecular weight of in each case at least 200 g/mol, particularly preferably at least 400 g/mol) on a linear main chain at more or less regular intervals. The lengths of these side chains are frequently approximately equal but may also differ greatly from one another (for example when polyether macromonomers having side chains of different lengths are incorporated in the form of polymerized units).
- In principle, component f) acts as accelerator and in a preferred embodiment contains an inorganic and an organic component. The inorganic component may be regarded as modified, finely disperse calcium silicate hydrate (C-S-H) which may contain foreign ions, such as magnesium and aluminium. The C-S-H can be prepared in the presence of the comb polymer plasticizer (organic component). Usually, a suspension containing the C-S-H in finely disperse form is obtained, which suspension firstly acts as a plasticizer and secondly effectively accelerates the hardening process of hydraulic binders.
- The inorganic component can in most cases be described with regard to its composition (not with regard to particle size, specific surface area, etc) by the following empirical formula:
-
a CaO, SiO2, b Al2O3, c H2O, d X, e W - X is an alkali metal
- W is an alkaline earth metal
-
0.1 ≦ a ≦ 2 preferably 0.66 ≦ a ≦ 1.7 0 ≦ b ≦ 1 preferably 0 ≦ b ≦ 0.1 1 ≦ c ≦ 6 preferably 1 ≦ c ≦ 6.0 0 ≦ d ≦ 1 preferably 0 ≦ d ≦ 0.4 0 ≦ e ≦ 2 preferably 0 ≦ e ≦ 0.1 - According to the present invention the C-S-H shows a calcium/silicium (Ca/Si)-molar ratio of 0.5 to 2.0, preferable 0.7 to 1.8, more preferable 1.6 to 1.7. The average particle size of C-S-H is smaller than 10 μm, preferable smaller than 1 μm, more preferable smaller than 0.2 μm, measured by light scattering with the equipment Master Sizer 2000 from the Malvern Company. In a further preferred embodiment the average particle size of C-S-H is greater 0.01 μm, preferable 0.1 μm to 1.0 μm, more preferable 0.2 μm to 0.5 μm.
- With other words the dispersant that is used for this method of preparation can be identical to the representatives of the dispersing component a) and/or b) of the formulation. The dispersing agent in this method of preparation is necessary for achieving a small particle size distribution of the C-S-H compound.
- Preferably that C-S-H containing composition is preperable by reaction of a calcium oxide, a calcium carbonate and/or a calcium hydroxide with a silicium dioxide during milling, the reaction being carried out in the presence of an aqueous solution that preferably contains a water-soluble copolymer that preferably is a dispersant for hydraulic binders and selected from at least a representative of component a) and/or b).
- In a further preferred embodiment of the invention, the water-soluble calcium compound is mixed in a first step, with the aqueous solution which contains a water-soluble comb polymer suitable as a plasticizer for hydraulic binders, so that a mixture preferably present as a solution is obtained, to which the water-soluble silicate compound is added in a subsequent second step.
- The aqueous solution may also contain one or more further solvents in addition to water.
- In a further preferred embodiment, the aqueous solution containing the dispersant and preferably one that is selected from component a) and/or b) furthermore has the water-soluble calcium compound and the water-soluble silicate compound as components dissolved in it.
- In general, the components used are used in the following ratios:
- i) 0.01 to 75, preferably 0.01 to 5, % by weight of water-soluble calcium compound,
- ii) 0.01 to 75, preferably 0.01 to 5% by weight of water-soluble silicate compound,
- iii) 0.001 to 60, preferably 0.1 to 15% by weight of water-soluble comb polymer suitable as a plasticizer for hydraulic binders (preferably component a) and/or b))
- iv) 24 to 99, preferably 90 to 99, % by weight of water.
- Frequently, the aqueous solution also contains, in addition to silicate and calcium ions, further dissolved ions which are preferably provided in the form of dissolved aluminium chloride and/or dissolved magnesium chloride.
- The water-soluble dispersant can be a comb polymer and be present as a copolymer which contains, on the back bone, side chains having ether functions and acid functions.
- As a rule, the water-soluble comb polymer is present as a copolymer which is produced by free radical polymerization in the presence of acid monomer and polyether macromonomer, so that altogether at least 45 mol %, preferably at least 80 mol %, of all structural units of the copolymer are produced by incorporation of acid monomer and polyether macromonomer in the form of polymerized units. Acid monomer is to be understood as meaning monomers which are capable of free radical copolymerization, have at least one carbon double bond, contain at least one acid function and react as an acid in an aqueous medium. Furthermore, acid monomer is also to be understood as meaning monomers which are capable of free radical copolymerization, have at least one carbon double bond, form at least one acid function in an aqueous medium as a result of a hydrolysis reaction and react as an acid in an aqueous medium (example: maleic anhydride).
- In the context of the present invention, polyether macromonomers are compounds which are capable of free radical copolymerization, have at least one carbon double bond, and have at least two ether oxygen atoms, with the proviso that the polyether macromonomer structural units present in the copolymer have side chains which contain at least two ether oxygen atoms.
- For further details reference is made to the description of the components a) and b) of the claimed formulation hereto.
- Often, the water-soluble calcium compound is present as calcium chloride, calcium nitrate, calcium formate, calcium acetate, calcium bicarbonate, calcium bromide, calcium carbonate, calcium citrate, calcium chlorate, calcium fluoride, calcium gluconate, calcium hydroxide, calcium hypochloride, calcium iodate, calcium iodide, calcium lactate, calcium nitrite, calcium oxalate, calcium phosphate, calcium propionate, calcium silicate, calcium stearate, calcium sulphate, calcium sulphate hemihydrate, calcium sulphate dihydrate, calcium sulphide, calcium tartrate and/or calcium aluminate, tricalcium silicate and/or dicalcium silicate.
- The water-soluble calcium compound is preferably present as calcium chloride, calcium nitrate and/or calcium formate.
- Often, the water-soluble silicate compound is present as sodium silicate, potassium silicate, waterglass, aluminium silicate, tricalcium silicate, dicalcium silicate, calcium silicate, silicic acid, sodium metasilicate and/or potassium metasilicate.
- The water-soluble silicate compound is preferably present as sodium metasilicate, potassium metasilicate and/or waterglass.
- In principle, a calcium silicate (provided that it is soluble) may be used both as a silicate source and as a calcium source. In many cases, however, this is not preferred. As a rule, species of different types are used as the water-soluble silicate compound and as the water-soluble calcium compound.
- According to the present invention the formulation is a liquid or a powder and preferably a redispersant powder.
- The powder form of the formulation can be achieved by any method known to a skilled person. Preferred is the spray drying method that is also suitable for getting the formulation of the invention as redispersant powder.
- Beside the formulation itself a method of use of the formulation states a further embodiment of the present invention.
- In this connection the use of the formulation for controlling the flowability of aqueous suspensions used in construction chemistry and in particular in aqueous suspensions containing hydraulic and/or latent hydraulic binders is of main interest. The formulation is used in particular as composition with dispersing properties. Regarding the aqueous suspensions it is a further embodiment that these compositions contain, as a hydraulic binder at least one representative selected from the group consisting of cements and calcium sulphate-based compounds, in particular calcium sulphate hemihydrate, anhydrite or gypsum. The aqueous suspension according to the present invention preferably is based on a dry mortar composition or a flooring composition. In a further embodiment the flooring composition contains calcium sulphate or cement or mixtures thereof, and preferably is a self-leveling flooring composition.
- Independent from the specific use the formulation according to the present invention is to be used in amounts of 0.001 to 8.0% by weight, in particular 0.005 to 5.0% by weight, preferably 0.01 to 2.0% by weight and particularly preferably 0.05 to 1.0% by weight, based in each case on the total composition of the suspension.
- Finally, the present invention comprises the option that the formulation is used together with other admixtures or compositions, preferably with flowability controlling and/or dispersing properties, and more preferably together with at least one dispersant of the type of component a) and/or the polymerisation product b) of the formulation.
- This means that the combination of component a) and component b) can be used as formulation according to the present invention and additionally that this formulation can be used together with other compounds, additives, admixtures or compositions. In consequence components a) and b) can be used as substantial constituents of the formulation and additionally as single compounds together with such formulation. This kind of use can be practiced stepwise, that means that either the formulation or the additional dispersants are added to the hydraulic binder containing composition in the first step of use and that additional amounts of the formulation its components are added in up following process steps.
- The following examples underline the advantages of the claimed formulation, its comprised components and its use.
- A reactor equipped with a stirrer and a heating mantle is filled with 600 parts of poly(ethyleneoxide)monophenylether (average molecular weight 5000 g/mol), 47.2 parts of concentrated methane sulfonic acid, 12 parts of water, 110 parts of α-phenyl-ω-hydroxypoly(oxy-1,2-ethanediyl)phosphate (average molecular weight 368 g/mol) and 14.7 parts of paraformaldehyde. This reaction mixture is stirred at 115° C. for 3 h. After cooling, 830 parts of water are added the reaction mixture is neutralized with 50% sodium hydroxide solution to a pH value of 6.5 to 7. The resin is a light yellow colored, clear and aqueous polymer solution with a solid concentration of 40% by weight. To the stirred solution (500 rpm) of the polymeric dispersant the antifoaming agent and the surfactant are added at ambient temperature (25° C.). The amounts of the materials shown in Table 2 are in percent by weight of the solution.
- A reactor equipped with a stirrer and a heating mantle is filled with 26 parts of polyphosphoric acid and heated to 90° C. Within 15 min 44.2 parts of 2-phenoxyethanol are charged into the reactor. After 1 h, 400 parts of poly(ethyleneoxide)monophenylether (average molecular weight 5000 g/mol), 31.4 parts of concentrated methane sulfonic acid, 20 parts of water and 12.6 parts of paraformaldehyde are added. This reaction mixture is stirred at 105° C. for 6 h. After cooling, 550 parts of water are added and the reaction mixture is neutralized with 50% sodium hydroxide solution to a pH value of 6.5 to 7. The resin is a light brown colored, clear and aqueous polymer solution with a solid concentration of 40% by weight. To the stirred solution (500 rpm) of the polymeric dispersant the antifoaming agent and the surfactant are added at ambient temperature (25° C.). The amounts of the materials shown in Table 2 are in percent by weight of the solution.
- A reactor equipped with a stirrer and a heating mantle is filled with 51.6 parts of polyphosphoric acid and heated to 90° C. Within 15 min 90 parts of 2-phenoxyethanol are charged into the reactor. After 1 h, 322 parts of poly(ethyleneoxide)monophenylether (average molecular weight 5000 g/mol), 300 parts of poly(ethyleneoxide)monophenylether (average molecular weight 2000 g/mol), 42.1 parts of concentrated methane sulfonic acid, 16.8 parts of water and 28.5 parts of paraformaldehyde are added. This reaction mixture is stirred at 105° C. for 6 h. After cooling, 800 parts of water are added and the reaction mixture is neutralized with 50% sodium hydroxide solution to a pH value of 6.5 to 7. The resin is a light brown colored, clear and aqueous polymer solution with a solid concentration of 40% by weight. To the stirred solution (500 rpm) of the polymeric dispersant the antifoaming agent and the surfactant are added at ambient temperature (25° C.). The amounts of the materials shown in Table 2 are in percent by weight of the solution.
- 2. Formulation of Dispersant Component a) with Polycondensate Component b)
- The examples E1 till E20 (see Table 1 and 2) were prepared by mixing the polycondensate components b) with equivalent amounts (wt. %) of the dispersants a). Melflux PCE 239 L 35% N.D., Melflux 2500 L 45% N.D., Melflux 2453 L 44% N.D., VP2661/493 L 40% N.D., Melflux 2424 L 50% N.D., Melflux AP 120 L 40%, and Sokalan DS5009 X are a polycarboxylate ether dispersant available from BASF Construction Polymers GmbH, Germany. Melcrete 500 L is a naphthalene sulfonate dispersant (BNS) available from BASF Construction Polymers GmbH. Melment L 15 G is a melamine sulphonate-formaldehyde condensate (MFS) available from BASF Construction Polymers GmbH. The non-ionic polymers N1 and N2 are able to maintain the fluidity of a cement composition and are synthesized according to the still unpublished application U.S. Ser. No. 12/477,637.
-
TABLE 1 Molar Ratio of Formulation polycondensate (E: Invention; Polycondensate b) and Solid Stability C: b) according to Dispersant dispersant content over 3 Comparison) example a) a) (wt. %) months E1 C7 Melflux PCE 2/1 35 stable 239 L E2 C8 Melflux PCE 2/1 35 stable 239 L E3 C7 BNS 1/1 25 stable E4 C8 BNS 1/1 25 stable E5 C9 BNS 1/1 25 stable E6 C8 Melflux 2500 L 1/1 40 stable E7-1 C8 VP2661/493 L 3/1 40 stable E7-2 C8 VP2661/493 L 1/3 40 stable E8-1 C8 Melflux PCE 3/1 35 stable 239 L E8-2 C8 Melflux PCE 1/3 35 stable 239 L E9 C8 BNS 2/1 20 stable E10 C8 Sokalan 2/1 35 stable 5009X E11 C8 Melflux AP 2/1 40 stable 120 L E12 C8 Melment L 15 G 2/1 40 stable E13 C8 N1 2/1 30 stable E14 C8 VP2661/493 L 2/1 40 stable E15 C9 VP2661/493 L 2/1 40 stable E16 C7 BNS 3/1 25 stable E17 C7 Melflux 2500 L 1/1 40 stable E18 C7 Melflux 2453 L 1/1 40 stable E19 C7 Melflux 2424 L 1/1 40 stable E20 C8 N2 2/1 30 stable C1 — Melflux 2500 L 40 C2 — Melflux PCE 35 239 L C3 — VP2661/493 L 40 C4 — 1:1 mixture 25 gel of Melflux formation 2500 L/BNS
3. Formulations of Polycondensates b), with Antifoaming Agents as Component a) - In the following admixtures (Test Solution) antifoaming agent A1 has been a polypropyleneglycol commercially available as Pluriol® P2000 and, antifoaming agent A2 an alkoxylated alcohol commercially available as Degressal® SD23 and antifoaming agent A3 a carboxylic ester commercially available as Degressal® SD30 all from BASF SE (Ludwigshafen, Germany). Surfactant S1 was an ethoxylated oxo-alcohol commercially available as Lutensol® TO6 from BASF SE (Ludwigshafen, Germany). Surfactant S2 (as component a) is a styrene/maleic acid copolymer which was synthesized according to EP 0306449 A2.
-
TABLE 2 Solution Dispersant Stability (E: Invention; according Antifoaming Surfactant over 3 C: to agent (wt.-%) (wt.-%) months Comparison) example A1 A2 A3 S1 S2 at RT E21 E1 0.4 0.6 + E22 1.1 0.2 0.3 + E23 1.1 0.4 0.6 + E24 E2 0.2 0.3 + E25 1.2 0.2 0.3 + E26 1.2 0.4 0.8 + E27 1.2 0.2 0.3 + E28 1.1 0.2 0.3 + E29 1.3 0.2 0.3 + E30 E1 0.2 0.3 + E31 E2 0.2 0.3 + C5 1.1 0.4 −)* C6 1.2 0.4 −)* C7 1.1 none none none none none n.a. C8 1.2 none none none none none n.a. C9 1.3 none none none none none n.a. )*phase separation within two days - The viscosities of the dispersants solutions were measured with a capillary viscometer at 25° C. The solutions were prepared by mixing a 25 Wt. % BNS solution with 25 Wt. % solutions of the dispersants as indicated in Table 1 and 3.
-
TABLE 3 Formulation Dispersant:BNS Viscosity in or dispersant ratio mPas C1 — 59.1 C4 1:1 529.4 C7 — 28.0 E3 1:1 137.1 C8 — 32.9 E4 1:1 188.8 C9 — 24.5 E5 1:1 83.2 - As can be seen from Table 3, the viscosity of the admixture E3, E4 and E5 is only slightly higher compared to the viscosity of the pure condensates C7, C8 and C9. Whereas the admixtures E3, E4 and E5 keep their low viscosity over time, the mixture of BNS and the polycarboxylate ether starts to form a non pourable gel. Unlike C4, the admixture E3, E4 and E5 are usable as dispersant agents for hydraulic binders (see below for application tests).
- 5. Gypsum based Thin Layer Levelling
-
Guide recipe: Alpha hemi hydrate 300 g Sand (0-0.2 mm) 350 g Lime stone filler 150 g Citric acid 0.10 g Starvis 3003 F 0.32 g Water 174.6 g Admixture (Superplasticizer) 0.15%-bws (=by weight of solids) - The required amounts of liquid admixture and water were weighted into a mixing cup. Afterwards the combined solids were added into the cup and mixed with a kitchen mixer for 60 sec at level two. The flow value was measured with a Vicat ring after 60 sec.
-
TABLE 4 Flow (cm) Admixture 5 min 20 min E26 25.7 27.2 No craters at the surface E25 29.5 30.5 No craters at the surface C8 28.6 30.2 Defects and crater
As illustrated in Table 4, the admixtures according to the invention show an excellent antifoaming property. The surface of thin layer levelling was found to be free of defects and craters compared to example C8. - The required amount of liquid admixture was weighted into the mixing cup and water was added to reach the water to stucco ratios given in Table 4. The stucco (400 g from various sources) together with the accelerator is sifted into water within 15 sec and afterwards mixed with a Hobart® mixer for 15 sec at high speed (285 rpm). After 60 sec the flow value was measured with a cylinder (height: 10 cm, diameter: 5 cm). The set time was determined by means of the so-called knife cut test.
-
TABLE 5 Natural stucco A Dosage Water to Accelerator)* Flow Set time Admixture [wt.-%] stucco ratio [g] [cm] [min:s] E26 0.050 0.63 0.400 20.6 2:20 E26 0.120 0.55 0.300 20.0 2:15 C8 0.055 0.63 0.400 20.1 2:10 C8 0.140 0.55 0.450 20.4 2:15 E31 0.050 0.63 0.400 20.4 2:10 E31 0.140 0.55 0.500 20.5 2:05 C2 0.050 0.63 0.500 20.6 2:10 C2 0.140 0.55 0.700 20.5 2:05 )*finely ground calcium sulfate dihydrate - As depicted in Table 5, the admixture E26 according to the invention show excellent dispersant abilities in comparison to the polycondensate dispersant C8. Admixture E31, which contains the polycondensate C8 and the polycarboxylate ether C2, displays a lower amount of accelerator usage as the pure polycarboxylate ether C2.
-
TABLE 6 Stucco from flue gas desulfurization Dosage Water to Accelerator)* Flow Set time Admixture [wt.-%] stucco ratio [g] [cm] [min:s] C8 0.190 0.53 0.060 20.0 2:15 E7-1 0.190 0.53 0.080 19.6 2:15 E7-2 0.190 0.53 0.150 20.1 2:20 C3 0.280 0.53 3.000 20.8 2:10 E8-1 0.190 0.53 0.080 22.6 2:20 E8-2 0.190 0.53 0.120 23.4 2:20 C2 0.190 0.53 0.120 20.2 2:15 E9 0.065 0.63 0.050 19.3 2:20 E10 0.065 0.63 0.060 22.9 2:20 E11 0.065 0.63 0.070 21.1 2:20 E12 0.065 0.63 0.050 19.8 2:20 C8 0.065 0.63 0.070 20.6 2:15 )*finely ground calcium sulfate dihydrate - As displayed in Table 6, the admixtures E7-1 and E7-2 according to the invention display excellent dispersant abilities in FGD stucco and in comparison to the polycarboxylate ether dispersant C3 a significant reduced accelerator demand. Admixtures E8-1 and E8-2 show higher flow values than the pure polycondensate C8. The admixtures E9 and E12, which contain the inexpensive dispersants BNS and MFS, exhibit at the same dosage as the polycondensate C8 good flow values.
-
TABLE 7 Natural clay containing stucco B Dosage Water to Accelerator)* Flow Set time Admixture [wt.-%] stucco ratio [g] [cm] [min:s] E26 0.280 0.60 0.230 20.8 2:00 E13 0.240 0.60 0.200 21.1 2:10 C3 0.280 0.60 — No flow — C2 0.280 0.755 0.280 20.7 2:15 )*finely ground calcium sulfate dihydrate - As shown in Table 7, the admixture according to the invention E26 and E13 possesses fluidity in the natural stucco B. At the same dosage, the comparison admixture C3 is not fluid whereas C2 needs a significant higher water to stucco ratio to reach the same flow value.
-
TABLE 8 Natural stucco C Dosage Water to Accelerator)* Flow Set time Admixture [wt.-%] stucco ratio [g] [cm] [min:s] C8 0.100 0.60 0.600 21.0 2:05 E26 0.100 0.60 0.800 22.3 2:05 E14 0.100 0.60 1.000 23.2 2:15 E15 0.100 0.60 0.700 23.5 2:20 C3 0.100 0.60 1.600 23.2 2:15 )*finely ground calcium sulfate dihydrate - As depicted in Table 8, the admixtures according to the invention display excellent dispersant abilities in the natural stucco C and in comparison to the polycarboxylate ether dispersant C3 reduced accelerator demands. The admixtures E26 and E15 have a similar low accelerator demand but a higher fluidity as the polycondensate C8.
- Water reduction and change of spread values over time in a mortar test system
- 600 g of cement powder is homogenized in a RILEM-Mixer. The required amount of water to reach the water to cement ratios given in Table 9 is added and mixed in for 30 sec at 140 rpm (level I). The sand is added during agitation via a funnel and mixed in for 30 sec at 140 rpm (level I). The brims of the bowl will be cleaned and the required amount of liquid admixture is added after a mixing break of 1.5 min. The mixing is continued for 60 sec at 285 rpm (level II) and afterwards the flow value (spread value) is determined with a Hagermann cylinder according to DIN EN 1015-3.
- The mortar is based on a Karistadt CEM I 42.5 R and has a sand to cement ratio of 2.2. The sand consists of a mixture of 30% quartz sand and 70% standardized sand.
-
TABLE 9 Spread Dosage value [% solid w/c @ 0 min 15 30 60 90 Example on cement] ratio [cm] min min min min blank — 0.545 24.6 23.5 23.5 23 C7 0.19 0.42 24.7 24.1 22.2 21.5 E16 0.27 0.42 25 23.4 22.2 20.5 BNS 0.50 0.42 24.9 23.6 22.5 21.2 E17 0.20 0.43 24.3 23.4 22.9 E18 0.20 0.40 25.0 25.5 24.9 24.5 E19 0.20 0.41 24.4 24.0 23.4 E13 0.23 0.43 23.5 23.8 25.4 25.2 24.4 E20 0.24 0.43 24.0 24.1 24.3 23.7 23.4 - The admixture E16, that contains the low-cost dispersant BNS, displays similar fluidity as the condensate C7 at a much lower dosage level than BNS. As FIG. 9 shows, the admixtures E18 and in particular E13 and E20 keep the fluidity of the cementious binder composition for more than 90 min.
Claims (88)
1-87. (canceled)
88. A formulation comprising
a) at least one component having dispersing properties and having been selected from the group consisting of a compound containing a branched comb polymer having polyether side chains, a naphthalene sulphonate-formaldehyde condensate and a melamine sulphonate-formaldehyde condensate; and
b) a polycondensation product containing
(I) at least one structural unit with an aromatic or heteroaromatic sub-unit and a polyether side chain and
(II) at least one phosphated structural unit with an aromatic or heteroaromatic sub-unit and
(III) at least one structural unit with an aromatic or heteroaromatic sub-unit,
structural unit (II) and structural unit (III) differing only in that the OP(OH)2 group of the structural unit (II) is replaced by H in structural unit (III), and structural unit (III) is not the same as structural unit (I).
89. A formulation according to claim 88 , wherein the component a) is a polycarboxylate ether a1), a polycarboxylate ester a2), an uncharged copolymer a3) or mixtures thereof.
90. A formulation according to either of claim 88 , wherein the component a) is a copolymer a1) comprising
1) at least one olefinically unsaturated moncarboxylic acid comonomer, or an ester or a salt thereof, or an olefinically unsaturated sulphonic acid comonomer or a salt thereof, and
2) at least one comonomer of the general formula (I)
and R2 is H or an aliphatic hydrocarbon radical having from 1 to 5 C atoms;
R3 is an unsubstituted or substituted aryl radical; and
R4 is H or an aliphatic hydrocarbon radical having 1 to 20 C atoms, a cycloaliphatic hydrocarbon radical having from 5 to 8 C atoms, a substituted aryl radical having 6 to 14 C atoms or a member selected from the groups consisting of
in which R5 and R7 are independently selected from the group consisting of an alkyl, aryl, aralkyl or alkaryl radical; and
R6 is an alkylidene, arylidene, aralkylidene or alkarylidene radical; and
p is from 0, 1, 2, 3 or 4,
m and n are independently selected from 2, 3, 4 or 5;
x and y are independently selected from an integer ≦350; and
z is from 0 to 200, wherein
(I) in copolymer a1), comonomer units which represent components 1) and 2) have no internal molecular differences or (II) the copolymer a,) is a polymeric mixture of the components 1) and 2) wherein the comonomer units have internal molecular differences with respect to at least one of R1, R2, R3, R4, R5, R6, R7, m, n, x, y, z, and the differences the composition and length of the side chains.
91. A formulation according to claim 89 , wherein the copolymer a1) contains the comonomer component 1) in a proportion of from 1 to 70 mol % and the comonomer component 2) in proportions of 99 to 30 mol %.
92. A formulation according to claim 89 , wherein the copolymer a1) contains the comonomer component 1) in proportions of 5 to 20 mol % and the comonomer component 2) in proportions of 40 to 90 mol %.
93. A formulation according to claim 88 , wherein the comonomer component 1) is an acrylic acid or a salt thereof, and the comonomer component 2) has a value of with p=0 or 1 and contains a vinyl or allyl group and R1 is a polyether.
94. A formulation according to claim 88 , wherein the comonomer component 1) is selected from the group consisting of acrylic acid, methacrylic acid, crotonic acid, isocrotonic acid, allylsulphonic acid, vinylsulphonic acid and suitable salts thereof and alkyl or hydroxyalkyl esters thereof.
95. A formulation according to claim 88 , wherein the copolymer a1) comprises additional structural groups in copolymerized form.
96. A formulation according to claim 95 , wherein the additional structural groups are styrenes, acrylamides or hydrophobic compounds, ester structural units, polypropylene oxide and polypropylene oxide/polyethylene oxide units being particularly preferred.
97. A formulation according to claim 95 , wherein the copolymer a1) contains the additional structural group in proportions up to 5 mol %.
98. A formulation according to claim 88 , wherein the formula (1) is a polyether containing allyl or vinyl groups.
99. A formulation according to claim 88 , wherein the polycarboxylate ester a2) is a polymer which is prepared by polymerization of a monomer mixture (I) containing in an amount of more than 50 wt. %, a representative of the carboxylic acid monomer type.
100. A formulation according to claim 99 , wherein the polycarboxylate ester a2) is an anti-foaming agent, a defoamer or a surfactant.
101. A formulation according to claim 99 , wherein the monomer mixture (I) contains an (alkoxy)polyalkylene glycol mono(meth)acrylate monomer (a) of the formula (II)
in which R1 is a hydrogen atom or a CH3 group, R2O is one representative or a mixture of at least two oxyalkylene groups having 2 to 4 carbon atoms, R3 is a hydrogen atom or an alkyl group having 1 to 5 carbon atoms and m is a number between 1 and 250 and is the average number of moles of the oxyalkylene group added,
additionally, as monomer (b), a (meth)acrylic acid of the general formula (III),
102. A formulation according to claim 101 , wherein the monomer (a) is present in an amount of 5 to 98% by weight, the monomer (b) in an amount of 2 to 95% by weight and the monomer (c) in an amount of up to 50% by weight in the monomer mixture (I), the respective amounts of the monomers (a), (b) and (c) summing to 100% by weight.
103. A formulation according to claim 99 , wherein the monomer (a) is a hydroxyethyl(meth)acrylate, hydroxypropyl(meth)acrylate, polyethylene glycol mono(meth)acrylate, polypropylene glycol mono(meth)acrylate, polybutylene glycol mono(meth)acrylate, polyethylene glycol polypropylene glycol mono(meth)acrylate, polyethylene glycol polybutylene glycol mono(meth)acrylate, polypropylene glycol polybutylene glycol mono(meth)acrylate, polyethylene glycol polypropylene glycol polybutylene glycol mono(meth)acrylate, methoxypolyethylene glycol mono(meth)acrylate, methoxypolypropylene glycol mono(meth)acrylate, methoxypolybutylene glycol mono(meth)acrylate, methoxypolyethylene glycol polypropylene glycol mono(meth)acrylate, methoxypolyethylene glycol polybutylene glycol mono(meth)acrylate, methoxypolypropylene glycol polybutylene glycol mono(meth)acrylate, methoxypolyethylene glycol polypropylene glycol polybutylene glycol mono(meth)acrylate, ethoxypolyethylene glycol mono(meth)acrylate, ethoxypolypropylene glycol mono(meth)acrylate, ethoxypolybutylene glycol mono(meth)acrylate, ethoxypolyethylene glycol polypropylene glycol mono(meth)acrylate, ethoxypolyethylene glycol polybutylene glycol mono(meth)acrylate, ethoxypolypropylene glycol polybutylene glycol mono(meth)acrylate, ethoxypolyethylene glycol polypropylene glycol polybutylene glycol mono(meth)acrylate or a mixture thereof.
104. A formulation according to claim 99 , wherein the monomer (b) is selected from the group consisting of acrylic acid, methacrylic acid, monovalent metal salts, divalent metal salts, ammonium salts and organic amine salts thereof and mixtures thereof.
105. A formulation according to claim 99 , wherein the monomer (c) is at least one representative of the esters of an aliphatic alcohol having 1 to 20 carbon atoms with an unsaturated carboxylic acid.
106. A formulation according to claim 105 , wherein the unsaturated carboxylic acid is maleic acid, fumaric acid, citraconic acid, (meth)acrylic acid or monovalent metal salts, divalent metal salts, ammonium salts or organic amine salts thereof.
107. A formulation according to claim 105 , said formula comprising monoesters or diesters of unsaturated dicarboxylic acids, such as maleic acid, fumaric acid or citraconic acid, with aliphatic C1-C20-alcohols, C2-C4-glycols or (alkoxy)polyalkylene glycols.
108. A formulation according to either of claim 88 , wherein the component a2) is a copolymer containing at least one of the following monomers:
A) an ethylenically unsaturated monomer comprising a hydrolyzable radical, hydrolyzable radical having an active bonding site for at least one of component a) or b);
B) an ethylenically unsaturated monomer having at least one C2-C4-oxyalkylene side group having a chain length of 1 to 30 units;
C) an ethylenically unsaturated monomer having at least one C2-C4-oxyalkylene side group having a chain length of 31 to 350 units.
109. A formulation according to claim 108 , wherein the components B) and C) are both present in the copolymer a2).
110. A formulation according to claim 108 , wherein the ethylenically unsaturated monomer of component A) comprises at least one anhydride, imide, maleic anhydride or maleimide.
111. A formulation according to claim 108 , wherein the ethylenically unsaturated monomer of component A) comprises an acrylate having an ester functionality which contains the hydrolyzable radical.
112. A formulation according to claim 111 , wherein the ester functionality is at least one hydroxypropyl or hydroxyethyl radical.
113. A formulation according to claim 108 , wherein the copolymer a2) in the component A) has more than one ethylenically unsaturated monomer with a hydrolyzable radical.
114. A formulation according to claim 113 , wherein the ethylenically unsaturated monomer of component A) has, as a radical, at least more than one ethylenically unsaturated monomer, wherein at least one is a hydrolyzable radical or a mixture of the two.
115. A formulation according to either of claims 113 , wherein the hydrolyzable radical has at least one C2-C20-alcohol functionality.
116. A formulation according to claim 113 , wherein the hydrolyzable radical is at least a C1-C20-alkyl ester, a C1-C20-aminoalkyl ester or an amide.
117. A formulation according to claim 108 , wherein at least one ethylenically unsaturated monomer of component B) or C) has a C2-C8-alkyl ether group.
118. A formulation according to claim 117 , wherein the ethylenically unsaturated monomer has a vinyl, allyl, or (methyl)allyl ether radical, or is derived from an unsaturated C2-C8-alcohol.
119. A formulation according to claim 118 , wherein the unsaturated C2-C8-alcohol is at least one representative of the series consisting of vinyl alcohol, (meth)allyl alcohol, isoprenol or methylbutenol.
120. A formulation according to claim 108 , wherein the ethylenically unsaturated monomer side groups of the component B) or C) have at least one C4-oxyalkylene unit.
121. A formulation according to claim 108 , wherein at least one ethylenically unsaturated monomer of the component B) or C) has a C2-C8-carboxylic ester which in particular is hydrolyzable.
122. A formulation according to claim 108 , wherein the oxyalkylene side groups have at least one ethylene oxide, one propylene oxide, one polyethylene oxide, one polypropylene oxide.
123. A formulation according to claim 108 , wherein the copolymer a2) in the component C) has at least one nonionic or one non-hydrolyzable monomer radical.
124. A formulation according to claim 88 , wherein the nonionic copolymer a3) is of formula (IV)
in which Q is an ethylenically unsaturated monomer having at least one hydrolyzable radical, G is O, C(O)—O or O—(CH2)p—O with p is from 2 to 8, mixtures of the variants of G being possible in a polymer; R1 and R2 are independently a C2-C8-alkyl;
R3 comprises (CH2)c, wherein c is an integer between 2 and 5, wherein mixtures of R3 may be present in the same polymer molecule;
R5 is at least one representative selected from the series consisting of H, a linear or branched saturated or unsaturated C1-C20-aliphatic hydrocarbon radical, a C5-C8-cycloaliphatic hydrocarbon radical or a substituted or unsubstituted C6-C14-aryl radical;
m is from 1 to 30;
n is 31 to 350;
w is 1 to 40;
y is from 0 to 1; and z is from 0 to 1;
wherein the sum (y+z) is >0.
126. A formulation according to claim 125 , wherein the hydrolyzable radical is at least one member of the group consisting of alkyl ester, aminoalkyl ester, hydroxylalkyl ester, aminohydroxyalkyl ester or amide.
127. A formulation according to either of claim 88 , wherein the nonionic copolymer a3) is at least one representative of the formula (VI)
128. A formulation according to claim 127 , wherein p is 4, R4 is C2H4OH or C3H6OH, each of the radicals R5 is H, m=5-30, n=31-250, w=1.5-30, y=0 to 1, z=0 to 1 and (y+z)>0.
129. A formulation according to claim 124 , wherein the molar ratio of w to the sum (y+z) is 1:1 to 20:1 and preferably 2:1 to 12:1.
130. A formulation according to claim 88 , wherein the copolymer a3) is a nonionic polyether-polyester copolymer.
131. A formulation according to claim 88 , wherein the structural units (I), (II), (III) of component b) have the following formulae
where
A are identical or different and are represented by substituted or unsubstituted aromatic or heteroaromatic compound having 5 to 10 C atoms,
where
B are identical or different and are represented by N, NH or O
where
n is 2, if B is N, and n is 1, if B is NH or O
where
R1 and R2 independently of one another, are identical or different and are represented by a branched or straight-chain C1- to C10-alkyl radical, C5- to C8-cycloalkyl radical, aryl radical, heteroaryl radical or H,
where
a are identical or different and are represented by an integer from 1 to 300,
wherein
X are identical or different and are represented by a branched or straight-chain C1- to C10-alkyl radical, C5- to C8-cycloalkyl radical, aryl radical, heteroaryl radical or H,
for (VIII) and (IX):
wherein
D are identical or different and are represented by a substituted or unsubstituted heteroaromatic compound having 5 to 10 C atoms;
wherein
E are identical or different and are represented by N, NH or O;
wherein
m is 2, if E is N, and m is 1,if E is NH or O;
wherein
R3 and R4 are independently selected from a branched or straight-chain C1- to C10-alkyl radical, C5- to C8-cycloalkyl radical, aryl radical, heteroaryl radical or H,
wherein b are identical or different and are represented by an integer from 0 to 300,
wherein
M is an alkaline metal ion, alkaline earth metal ion, ammonium ion, organic ammonium ion or H;
c is 1 or ½, wherein if M is an alkaline earth metal ion, c is ½.
132. A formulation according to claim 131 , wherein the component b) contains a structural unit (X) of formula
wherein
Y, independently of one another, are identical or different and are represented by (VII), (VIII), (IX) or further constituents of the polycondensation product b),
wherein
R5 are identical or different and are represented by H, CH3, COOMc or a substituted or unsubstituted aromatic or heteroaromatic compound having 5 to 10 C atoms,
wherein
R6 are identical or different and are represented by H, CH3, COOMc or a substituted or unsubstituted aromatic or heteroaromatic compound having 5 to 10 C atoms,
wherein
M is independently of one another an alkaline metal ion, alkaline earth metal ion, ammonium ion, organic ammonium ion or H,
c is 1 or ½, wherein if M is alkaline earth metal ion c is ½.
133. A formulation according to claim 132 , wherein R5 and R6 in structural unit (X) of component b), independently of one another, are identical or different and are represented by H, COOMc or methyl.
134. A formulation according to claim 88 , wherein the molar ratio of the structural units [(VII)+(VII)+(IX)]:(X) in component b) is 1:0.8 to 3.
135. A formulation according to claim 88 , wherein the molar ratio of the structural units (VII):[(VIII)+(IX)] in component b) is 1:15 to 15:1 and preferably 1:10 to 10:1.
136. A formulation according to claim 88 , wherein the molar ratio of the structural units (VII):(IX) in component b) is 1:0.005 to 1:10.
137. A formulation according to claim 88 , wherein the polycondensation product b) is present in aqueous solution which contains 2 to 90% by weight of water and 98 to 10% by weight of dissolved dry matter.
138. A formulation according to claim 88 , wherein the component a) is present in proportions of 5 to 95% by weight based in each case on the total formulation.
139. A formulation according to claim 88 , wherein the component b) is present in proportions of 5 to 100% by weight based in each case on the total formulation.
140. A formulation according to claim 88 , further comprising at least one antifoaming agent as component c) or a component d) having a surface-active effect, wherein the components c) and d) are structurally different from one another.
141. A formulation according to claim 140 , wherein the antifoam component c) is a member selected from the group consisting of mineral oil, vegetable oil, silicone oil, silicone-containing emulsions, fatty acid, fatty acid ester, organically modified polysiloxane, borate ester, alkoxylate, polyoxyalkylene copolymer, ethylene oxide (EO)-propylene oxide (PO) block polymer, acetylenic diol having antifoam properties, phosphoric acid ester of the formula P(O)(O—R8)3-x(O—R9)x wherein P is phosphorus, O is oxygen and R8 and R9 independently of one another, is a C2-20-alkyl or an aryl group and x is 0, 1 or 2.
142. A formulation according to claim 140 , wherein the antifoaming component c) is at least one member selected from the group consisting of trialkyl phosphate, polyoxypropylene copolymer and glycerol/alcohol acetate.
143. A formulation according to claim 140 , wherein the antifoaming component c) is triisobutyl phosphate.
144. A formulation according to claim 140 , wherein the antifoam component c) is a mixture of a trialkylphosphate and a polyoxypropylene copolymer.
145. A formulation according to claim 140 , wherein the component d) is at least one representative of the series consisting of ethylene oxide/propylene oxide block copolymer, styrene/maleic acid copolymer, fatty acid alcohol alkoxylate, alcohol ethoxylate R10((EO)(H wherein R10 is an aliphatic hydrocarbon group having 1 to 25 carbon atoms, acetylenic diol, monoalkylpolyalkylene, ethoxylated nonylphenol, alkyl sulphate, alkyl ether sulphate, alkyl ether sulphonate or alkyl ether carboxylate.
146. A formulation according to either of claim 140 , wherein the component d) comprises an alcohol having a polyalkylene group, the polyalkylene group having a carbon chain length of 2 to 20 carbon atoms.
147. A formulation according to claim 146 , wherein the polyalkylene group has a carbon chain length of 3 to 12 carbon atoms.
148. A formulation according to claim 140 , wherein it contains the antifoaming component c) in free form, bound to the dispersant component a) or as a mixture of these two forms.
149. A formulation according to claim 140 , wherein the antifoaming component c) is present in an amount of from 0.01 to 10% by weight or the surface-active component d) is present in an amount of from 0.01 to 10% by weight, based in each case on the total weight of the formulation.
150. A formulation according to claim 140 , wherein the antifoaming c) or the surface-active component d), independently of one another, are present in each case in an amount of 0.01 to 5% by weight, based in each case on the total weight of the formulation.
151. A formulation according to claim 88 , wherein component e) is a compound selected from the group consisting of a polymer having a low charge, neutral polymer and polyvinyl alcohol as component e).
152. A formulation according to claim 151 , wherein the component e) is present in an amount of from 1 to 50% by weight based in each case on the total weight of the formulation.
153. A formulation according to claim 151 , wherein the polymer having a low charge is branched and the side chain preferably consists of a polyether or a polyester.
154. A formulation according to claim 151 , wherein the polymer having a low charge is at least one of a polycarboxylate ether or a polycarboxylate ester.
155. A formulation according to claim 151 , wherein the polymer e) having a low charge is composed of at least one monomer selected from the series consisting of polyether monoacrylate, polyether monomethacrylate, polyether monoallyl ether, polyether monomaleate, monovinylated polyether or mixtures thereof.
156. A formulation according to claim 155 , wherein the polyether is an alkylene oxide polymer having a molecular weight of 500 to 10,000.
157. A formulation according to claim 156 , wherein the alkylene oxide is ethylene oxide, propylene oxide, butylene oxide or a mixture thereof.
158. A formulation according to claim 151 , wherein the polymer e) having a low charge is composed of at least one monomer selected from the series consisting of polypropylene glycol acrylate, polypropylene glycol methacrylates, polyethylene glycol acrylate, polyethylene glycol methacrylate, polypropylene glycol monovinyl ether, polyethylene glycol monovinyl ether, alkoxy- or aryloxypolyethylene glycol acrylate, alkoxy- or aryloxypolyethylene glycol methacrylates, alkoxy- or aryloxypolyethylene glycol monovinyl ether, acrylates, methacrylates and monovinyl ethers of an oxyalkylene or oxypropylene block or random copolymer, polypropylene glycol allyl ether, polyethylene glycol allyl ether, polyethylene glycol monomaleate polypropylene glycol monomaleate and any mixtures thereof.
159. A formulation according to claim 151 , wherein the polymer e) having a low charge carries a carboxylic acid group, preferably selected from the series consisting of acrylic acid, methacryl acid, maleic acid, fumaric acid, itaconic acid or anhydrides thereof.
160. A formulation according to claim 151 , wherein the polymer e) having a low charge carries a sulphonic acid group selected from the series consisting of 2-acrylamido-2-methylpropanesulphonic acid (AMPS), vinylsulphonic acid, allyl ether sulphonic acid, 2-sulphoethylmethacrylic acid, styrenesulphonic acid, methallylsulphonic acid, the sodium, potassium and ammonium salts thereof and any mixtures thereof, and in particular the AMPS and vinylsulphonic acid.
161. A formulation according to claim 151 , wherein the neutral polymer e) is composed of neutral monomer building blocks which are selected in particular from the series consisting of alkyl acrylates and alkyl methacrylates and hydroxyalkyl esters thereof having up to 5 carbon atoms, in particular hydroxyethyl acrylate and hydroxypropyl acrylate or hydroxyethyl methacrylate and hydroxypropyl methyacrylate, and vinyl acetate, N-vinylpyrrolidone, N-vinylcaprolactam, styrene and methylstyrene.
162. A formulation according to claim 88 , further comprising a component f) which is a calcium-silicate-hydrate (C-S-H) containing composition.
163. A formulation according to claim 162 , wherein the C-S-H shows a calcium/silicium (Ca/Si)-molar ratio of 0.5 to 2.0.
164. A formulation according to claim 162 , wherein the average particle size of C-S-H is smaller than 10 μm when measured by light scattering with the equipment Master Sizer 2000 from the Malvern Company.
165. A formulation according to claim 162 , wherein the average particle size of C-S-H is greater 0.01 μm.
166. A formulation according to claim 162 , wherein the C-S-H containing composition is prepareble by reaction of a water-soluble calcium containing compound with a water-soluble silicate containing compound, the reaction of the water-soluble calcium containing compound with the water-soluble silicate containing compound being carried out in the presence of an aqueous solution preferably containing a water-soluble copolymer that preferably is a dispersant for hydraulic binders and selected from at least a representative of at least one of component a) or b).
167. A formulation according to claims 162 , wherein the C-S-H containing composition is prepareble by reaction of a calcium oxide, a calcium carbonate and/or a calcium hydroxide with a silicium dioxide during milling, the reaction being carried out in the presence of an aqueous solution that preferably contains a water-soluble copolymer that preferably is a dispersant for hydraulic binders and selected from at least a representative of at least one of component a) or b).
168. A formulation according to claim 88 , wherein it is a liquid or a powder.
169. A method of controlling the flowability of an aqueous comprising at least one of hydraulicor latent hydraulic binder comprising adding a sufficient the formulation of claim 88 to an aqueous suspension of said hydraulic binder or said latent hydraulic binder to control the flowability of the aqueous suspension.
170. A method according to claim 169 , wherein the hydraulic binder is at least one of a cement or a calcium sulphate-based.
171. A method according to claim 169 , wherein the formulation is present in an amount of from 0.001 to 8.0% by weight based on the total composition of the suspension.
172. A method according to claim 169 , wherein the formulation further comprises an additive, an admixture or a composition with at least one of flowability controlling or dispersing properties.
173. A method according to claim 169 wherein the aqueous suspension comprises a dry mortar composition or a flooring composition.
174. A method according to claim 173 , wherein the flooring composition contains calcium sulphate, cement or a mixture thereof, and preferably is a self-leveling flooring composition.
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US12/552,338 US20110054081A1 (en) | 2009-09-02 | 2009-09-02 | Formulation and its use |
| US13/611,486 US20130005861A1 (en) | 2009-09-02 | 2012-09-12 | Formulation and its use |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US23925909P | 2009-09-02 | 2009-09-02 | |
| US12/552,338 US20110054081A1 (en) | 2009-09-02 | 2009-09-02 | Formulation and its use |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/611,486 Division US20130005861A1 (en) | 2009-09-02 | 2012-09-12 | Formulation and its use |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20110054081A1 true US20110054081A1 (en) | 2011-03-03 |
Family
ID=43625790
Family Applications (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/552,338 Abandoned US20110054081A1 (en) | 2009-09-02 | 2009-09-02 | Formulation and its use |
| US12/870,381 Active 2031-04-02 US8597426B2 (en) | 2009-09-02 | 2010-08-27 | Additives in gypsum panels and adjusting their proportions |
| US13/611,486 Abandoned US20130005861A1 (en) | 2009-09-02 | 2012-09-12 | Formulation and its use |
| US14/075,880 Active US8801852B2 (en) | 2009-09-02 | 2013-11-08 | Additives in gypsum slurries and adjusting their proportions |
Family Applications After (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US12/870,381 Active 2031-04-02 US8597426B2 (en) | 2009-09-02 | 2010-08-27 | Additives in gypsum panels and adjusting their proportions |
| US13/611,486 Abandoned US20130005861A1 (en) | 2009-09-02 | 2012-09-12 | Formulation and its use |
| US14/075,880 Active US8801852B2 (en) | 2009-09-02 | 2013-11-08 | Additives in gypsum slurries and adjusting their proportions |
Country Status (6)
| Country | Link |
|---|---|
| US (4) | US20110054081A1 (en) |
| AR (1) | AR078255A1 (en) |
| CA (1) | CA2773145C (en) |
| MX (1) | MX2012002325A (en) |
| TW (1) | TW201116501A (en) |
| WO (1) | WO2011028817A1 (en) |
Cited By (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20120172468A1 (en) * | 2010-12-30 | 2012-07-05 | Blackburn David R | Effective use of melamine sulfonate condensate dispersants in wallboard containing foam |
| CN103011680A (en) * | 2012-12-13 | 2013-04-03 | 中国矿业大学(北京) | Sulfate-resistant organosilicone modified polycarboxylic superplasticizer and preparation method thereof |
| US20130203926A1 (en) * | 2012-02-08 | 2013-08-08 | Bogdan Moraru | Aqueous dispersion |
| WO2013117465A1 (en) * | 2012-02-08 | 2013-08-15 | Basf Se | Aqueous dispersion |
| US20140135427A1 (en) * | 2011-08-10 | 2014-05-15 | Sika Technology Ag | Process for drying concrete dispersants |
| US20140134407A1 (en) * | 2008-05-06 | 2014-05-15 | Intertape Polymer Corporation | Edge coating for tapes |
| CN103922637A (en) * | 2014-03-14 | 2014-07-16 | 石家庄市长安育才建材有限公司 | Water reducing agent for high performance C80 concrete, and preparation method and application method thereof |
| WO2016069289A1 (en) | 2014-10-31 | 2016-05-06 | Rohm And Haas Company | Two component synthetic water retention agent and rheology modifier for use in cements, mortars and plasters |
| CN107383358A (en) * | 2017-07-17 | 2017-11-24 | 上海台界化工有限公司 | Hyperbranched synthetic method of unsaturated APEO and products thereof and application |
| CN108417836A (en) * | 2018-01-31 | 2018-08-17 | 闽南师范大学 | A kind of binders for electrodes of new type lithium ion battery and preparation method thereof |
| RU2689166C1 (en) * | 2013-12-20 | 2019-05-24 | Констракшн Рисёрч Энд Текнолоджи Гмбх | Additive for improvement of rheological properties of inorganic binding substances |
| WO2020133777A1 (en) * | 2018-12-24 | 2020-07-02 | 科之杰新材料集团有限公司 | Phosphate type polycarboxylate water reducer and preparation method |
| CN111377645A (en) * | 2018-12-31 | 2020-07-07 | 江苏苏博特新材料股份有限公司 | Micromolecular phosphonic acid water reducing agent suitable for machine-made sand and preparation method thereof |
| CN111433171A (en) * | 2017-12-07 | 2020-07-17 | 建筑研究和技术有限公司 | Dispersant composition |
| CN111533844A (en) * | 2020-06-04 | 2020-08-14 | 江苏万邦新材料科技有限公司 | Modified concrete water-retaining agent and preparation method thereof |
| CN111925488A (en) * | 2020-08-11 | 2020-11-13 | 陈兴涛 | Shrinkage-reducing polycarboxylate superplasticizer and preparation method thereof |
| CN112313186A (en) * | 2018-06-21 | 2021-02-02 | 竹本油脂株式会社 | Additive for hydraulic composition |
| CN113882155A (en) * | 2020-07-01 | 2022-01-04 | 中国石油化工股份有限公司 | Polyethylene glycol maleimide-styrene copolymer sizing agent and preparation method and application thereof |
Families Citing this family (49)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9802866B2 (en) | 2005-06-09 | 2017-10-31 | United States Gypsum Company | Light weight gypsum board |
| USRE44070E1 (en) | 2005-06-09 | 2013-03-12 | United States Gypsum Company | Composite light weight gypsum wallboard |
| US9840066B2 (en) * | 2005-06-09 | 2017-12-12 | United States Gypsum Company | Light weight gypsum board |
| US7731794B2 (en) * | 2005-06-09 | 2010-06-08 | United States Gypsum Company | High starch light weight gypsum wallboard |
| US11306028B2 (en) | 2005-06-09 | 2022-04-19 | United States Gypsum Company | Light weight gypsum board |
| US11338548B2 (en) | 2005-06-09 | 2022-05-24 | United States Gypsum Company | Light weight gypsum board |
| HUE034354T2 (en) * | 2009-11-16 | 2018-02-28 | Bpb Ltd | Plaster-based material including an agent capable of trapping formaldehyde. |
| CN107082618A (en) * | 2010-10-11 | 2017-08-22 | 巴斯夫聚合建材有限公司 | Gypsum slurry containing dispersant |
| US9296124B2 (en) | 2010-12-30 | 2016-03-29 | United States Gypsum Company | Slurry distributor with a wiping mechanism, system, and method for using same |
| WO2012092582A1 (en) | 2010-12-30 | 2012-07-05 | United States Gypsum Company | Slurry distributor, system and method for using same |
| US9999989B2 (en) | 2010-12-30 | 2018-06-19 | United States Gypsum Company | Slurry distributor with a profiling mechanism, system, and method for using same |
| NZ613438A (en) | 2010-12-30 | 2015-05-29 | United States Gypsum Co | Slurry distribution system and method |
| US10076853B2 (en) | 2010-12-30 | 2018-09-18 | United States Gypsum Company | Slurry distributor, system, and method for using same |
| US8323785B2 (en) | 2011-02-25 | 2012-12-04 | United States Gypsum Company | Lightweight, reduced density fire rated gypsum panels |
| JP6246722B2 (en) | 2011-10-24 | 2017-12-13 | ユナイテッド・ステイツ・ジプサム・カンパニー | Multi-leg discharge boot for cementitious aqueous slurry dispensing, gypsum slurry mixing and dispensing assembly, and method of compounding cementitious products |
| MX353809B (en) | 2011-10-24 | 2018-01-30 | United States Gypsum Co | Multi-piece mold and method of making slurry distributor. |
| MX353810B (en) | 2011-10-24 | 2018-01-30 | United States Gypsum Co | Flow splitter for slurry distribution system. |
| EP2592054B1 (en) | 2011-11-11 | 2014-11-05 | Omya International AG | Aqueous suspensions of calcium carbonate-comprising materials with low deposit built up |
| AU2013221770B2 (en) | 2012-02-17 | 2016-06-09 | United States Gypsum Company | Gypsum products with high efficiency heat sink additives |
| TR201808481T4 (en) * | 2012-04-11 | 2018-07-23 | Construction Research & Technology Gmbh | Polycondensation product based on aromatic compounds, method for its production and its use. |
| EP2684999B1 (en) | 2012-07-13 | 2014-12-17 | Omya International AG | High solids and low viscous aqueous slurries of calcium carbonate-comprising materials with improved rheological stability under increased temperature |
| US9828441B2 (en) | 2012-10-23 | 2017-11-28 | United States Gypsum Company | Method of preparing pregelatinized, partially hydrolyzed starch and related methods and products |
| US9540810B2 (en) | 2012-10-23 | 2017-01-10 | United States Gypsum Company | Pregelatinized starch with mid-range viscosity, and product, slurry and methods related thereto |
| US10399899B2 (en) | 2012-10-23 | 2019-09-03 | United States Gypsum Company | Pregelatinized starch with mid-range viscosity, and product, slurry and methods related thereto |
| CA2895797C (en) * | 2012-12-20 | 2017-09-26 | Georgia-Pacific Gypsum Llc | Hydroxide-mediated hydrophobing compositions for making water-resistant gypsum board |
| DE202012012306U1 (en) * | 2012-12-21 | 2013-01-16 | Knauf Insulation Gmbh | Wood wool board with specific plaster |
| KR102021857B1 (en) * | 2013-07-23 | 2019-09-17 | 엘지전자 주식회사 | Mobile terminal and panorama capturing method thereof |
| EP2848597A1 (en) * | 2013-09-17 | 2015-03-18 | Basf Se | Light-weight gypsum board with improved strength and method for making same |
| US10189180B2 (en) | 2014-01-15 | 2019-01-29 | United States Gypsum Company | Foam injection system with variable port inserts for slurry mixing and dispensing apparatus |
| US10059033B2 (en) | 2014-02-18 | 2018-08-28 | United States Gypsum Company | Cementitious slurry mixing and dispensing system with pulser assembly and method for using same |
| CN106661613B (en) | 2014-05-13 | 2020-12-08 | 生命科技股份有限公司 | System and method for validating sequencing results |
| US10309771B2 (en) | 2015-06-11 | 2019-06-04 | United States Gypsum Company | System and method for determining facer surface smoothness |
| US11040513B2 (en) | 2015-06-24 | 2021-06-22 | United States Gypsum Company | Composite gypsum board and methods related thereto |
| US10407344B2 (en) | 2015-10-01 | 2019-09-10 | United States Gypsum Company | Foam modifiers for gypsum slurries, methods, and products |
| US10662112B2 (en) | 2015-10-01 | 2020-05-26 | United States Gypsum Company | Method and system for on-line blending of foaming agent with foam modifier for addition to cementitious slurries |
| CN105254205A (en) * | 2015-10-06 | 2016-01-20 | 李康 | Concrete slump remaining agent and preparation method thereof |
| JP6873136B2 (en) * | 2015-12-17 | 2021-05-19 | コンストラクション リサーチ アンド テクノロジー ゲーエムベーハーConstruction Research & Technology GmbH | Water reducing agent based on polycondensate |
| CN105646871A (en) * | 2015-12-31 | 2016-06-08 | 江苏苏博特新材料股份有限公司 | Preparation method of polymer and application thereof |
| US20170326840A1 (en) | 2016-05-13 | 2017-11-16 | United States Gypsum Company | Method for preparing mat-faced board |
| US10442732B2 (en) | 2016-05-20 | 2019-10-15 | United States Gypsum Company | Gypsum slurries with linear polycarboxylate dispersants |
| US9878950B1 (en) * | 2016-07-11 | 2018-01-30 | National Gypsum Properties, Llc | Liquid gypsum set accelerator |
| US11225046B2 (en) | 2016-09-08 | 2022-01-18 | United States Gypsum Company | Gypsum board with perforated cover sheet and system and method for manufacturing same |
| US10752558B2 (en) * | 2017-11-20 | 2020-08-25 | Continental Building Products Operating Company, LLC | System and method for utilizing canister and hose to move slurry mixture to make gypsum board |
| CN108102103B (en) * | 2017-12-04 | 2020-12-29 | 江苏苏博特新材料股份有限公司 | Preparation method and application of micromolecular phosphate additive |
| CN108751785B (en) * | 2018-07-27 | 2020-12-08 | 石家庄市长安育才建材有限公司 | Nano crystal seed early strength agent and preparation method thereof |
| CN109021188B (en) * | 2018-08-29 | 2021-04-20 | 江苏苏博特新材料股份有限公司 | Modified phenolic aldehyde amine polycondensate cement grinding aid, and preparation method and application thereof |
| CN109293885B (en) * | 2018-09-28 | 2021-04-20 | 镇江苏博特新材料有限公司 | Phosphate group-containing water reducing agent and preparation method thereof |
| CN109796152A (en) * | 2019-03-04 | 2019-05-24 | 淮北旭日建材有限公司 | A kind of production method and its Preparation equipment of QS-2 efficient retarding and water reducing agent |
| WO2023205049A1 (en) | 2022-04-20 | 2023-10-26 | United States Gypsum Company | Gypsum set accelerator |
Citations (26)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3686133A (en) * | 1969-08-21 | 1972-08-22 | Kao Corp | Dispersing composition |
| US4501839A (en) * | 1981-03-02 | 1985-02-26 | Sika Ag, Vorm. Kaspar Winkler & Co. | Method of using highly concentrated aqueous solutions of low viscosity of melamine/aldehyde resins for improving building materials |
| US4725665A (en) * | 1985-08-23 | 1988-02-16 | Chemie Linz Aktiengesellschaft | Use of salts of water-soluble |
| US5158996A (en) * | 1987-08-28 | 1992-10-27 | Sandoz Ltd. | Chemically treated anhydride copolymers and cementitious mixtures containing the copolymers |
| US5750634A (en) * | 1995-03-17 | 1998-05-12 | Skw Trostberg Aktiengesellschaft | Water-soluble polycondensation products based on amino-s-triazines and the use thereof |
| US5798425A (en) * | 1995-04-07 | 1998-08-25 | Skw Trostberg Aktiengesellschaft | Co-polymers based on oxyalkyleneglycol alkenyl ethers and unsaturated dicarboxylic acid derivatives |
| US6139623A (en) * | 1997-01-21 | 2000-10-31 | W. R. Grace & Co.-Conn. | Emulsified comb polymer and defoaming agent composition and method of making same |
| US6187941B1 (en) * | 1997-09-06 | 2001-02-13 | Asta Medica Aktiengesellschaft | Process for the preparation of oxazaphosphorine-2-amines |
| US6506248B1 (en) * | 1997-04-10 | 2003-01-14 | James Hardie Research Pty Limited | Building products |
| US6555683B1 (en) * | 1995-03-31 | 2003-04-29 | Skw Polymers Gmbh | Condensation products based on amino-s-triazines and the use thereof |
| US6777517B1 (en) * | 1999-06-11 | 2004-08-17 | Degussa Construction Chemicals Gmbh | Copolymers based on unsaturated mono-or dicarboxylic acid derivatives and oxyalkylene glycol alkenyl ethers, method for the production and use thereof |
| US20050239924A1 (en) * | 2002-03-27 | 2005-10-27 | Lettkeman Dennis M | High molecular weight additives for calcined gypsum and cementitious compositions |
| US20050250888A1 (en) * | 2002-03-27 | 2005-11-10 | Lettkeman Dennis M | High strength flooring compositions |
| US7041167B2 (en) * | 2001-03-05 | 2006-05-09 | James Hardie International Finance B.V. | Low density accelerant and strength enhancing additive for cementitious products and methods of using same |
| US7070648B1 (en) * | 2005-06-16 | 2006-07-04 | Lyondell Chemical Technology, L.P. | Preparation of gypsum compositions |
| US20060278130A1 (en) * | 2005-06-14 | 2006-12-14 | Qingxia Liu | Gypsum products utilizing a two-repeating unit dispersant and a method for making them |
| US20060281886A1 (en) * | 2005-06-14 | 2006-12-14 | Manfred Bichler | Polyether-containing copolymer |
| US20060278134A1 (en) * | 2005-06-14 | 2006-12-14 | Lettkeman Dennis M | Modifiers for gypsum slurries and method of using them |
| US20060278129A1 (en) * | 2005-06-14 | 2006-12-14 | Qingxia Liu | Effective use of dispersants in wallboard containing foam |
| US20060280970A1 (en) * | 2005-06-14 | 2006-12-14 | United States Gypsum Company | High strength flooring compositions |
| US20060281837A1 (en) * | 2005-06-14 | 2006-12-14 | United States Gypsum Company | Fast drying gypsum products |
| US20060280899A1 (en) * | 2005-06-14 | 2006-12-14 | United States Gypsum Company | Method of making a gypsum slurry with modifiers and dispersants |
| US20080017078A1 (en) * | 2005-06-14 | 2008-01-24 | Manfred Bichler | Liquid admixture composition |
| US20080108732A1 (en) * | 2004-10-15 | 2008-05-08 | Philipp Wieland | Polycondensation Product Base on Aromatic or Heteroaromatic Compounds, Method for the Production Thereof, and Use Thereof |
| US20090101045A1 (en) * | 2007-10-23 | 2009-04-23 | Dennis Lettkeman | Gypsum mixtures for forming solids |
| US20100197840A1 (en) * | 2007-07-31 | 2010-08-05 | Sika Technology Ag | Emulsifying polymers and their use |
Family Cites Families (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2078199A (en) | 1936-10-02 | 1937-04-20 | United States Gypsum Co | Heatproofed set-stabilized gypsum plaster |
| US3573947A (en) | 1968-08-19 | 1971-04-06 | United States Gypsum Co | Accelerator for gypsum plaster |
| DE4411797A1 (en) | 1994-04-06 | 1995-10-12 | Sueddeutsche Kalkstickstoff | Sulphanilic acid-contg. melamine-formaldehyde] condensate prepn. |
| CA2158820C (en) * | 1994-09-23 | 2004-11-23 | Steven W. Sucech | Producing foamed gypsum board |
| MY114306A (en) | 1995-07-13 | 2002-09-30 | Mbt Holding Ag | Cement dispersant method for production thereof and cement composition using dispersant |
| US5683635A (en) | 1995-12-22 | 1997-11-04 | United States Gypsum Company | Method for preparing uniformly foamed gypsum product with less foam agitation |
| US6342284B1 (en) | 1997-08-21 | 2002-01-29 | United States Gysum Company | Gypsum-containing product having increased resistance to permanent deformation and method and composition for producing it |
| US6632550B1 (en) | 1997-08-21 | 2003-10-14 | United States Gypsum Company | Gypsum-containing product having increased resistance to permanent deformation and method and composition for producing it |
| US6409825B1 (en) | 2000-11-22 | 2002-06-25 | United States Gypsum Company | Wet gypsum accelerator and methods, composition, and product relating thereto |
| US6893752B2 (en) | 2002-06-28 | 2005-05-17 | United States Gypsum Company | Mold-resistant gypsum panel and method of making same |
| DE102006027035A1 (en) | 2005-06-14 | 2007-01-11 | Basf Construction Polymers Gmbh | Polyether-containing copolymer |
| US20060278128A1 (en) | 2005-06-14 | 2006-12-14 | United States Gypsum Company | Effective use of dispersants in wallboard containing foam |
| US8088218B2 (en) * | 2005-06-14 | 2012-01-03 | United States Gypsum Company | Foamed slurry and building panel made therefrom |
| US20080148997A1 (en) * | 2006-12-22 | 2008-06-26 | Blackburn David R | Gypsum compositions with naphthalene sulfonate and modifiers |
-
2009
- 2009-09-02 US US12/552,338 patent/US20110054081A1/en not_active Abandoned
-
2010
- 2010-08-27 US US12/870,381 patent/US8597426B2/en active Active
- 2010-09-01 AR ARP100103211A patent/AR078255A1/en unknown
- 2010-09-01 TW TW099129452A patent/TW201116501A/en unknown
- 2010-09-01 WO PCT/US2010/047551 patent/WO2011028817A1/en active Application Filing
- 2010-09-01 CA CA2773145A patent/CA2773145C/en active Active
- 2010-09-01 MX MX2012002325A patent/MX2012002325A/en active IP Right Grant
-
2012
- 2012-09-12 US US13/611,486 patent/US20130005861A1/en not_active Abandoned
-
2013
- 2013-11-08 US US14/075,880 patent/US8801852B2/en active Active
Patent Citations (29)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3686133A (en) * | 1969-08-21 | 1972-08-22 | Kao Corp | Dispersing composition |
| US4501839A (en) * | 1981-03-02 | 1985-02-26 | Sika Ag, Vorm. Kaspar Winkler & Co. | Method of using highly concentrated aqueous solutions of low viscosity of melamine/aldehyde resins for improving building materials |
| US4725665A (en) * | 1985-08-23 | 1988-02-16 | Chemie Linz Aktiengesellschaft | Use of salts of water-soluble |
| US5158996A (en) * | 1987-08-28 | 1992-10-27 | Sandoz Ltd. | Chemically treated anhydride copolymers and cementitious mixtures containing the copolymers |
| US5750634A (en) * | 1995-03-17 | 1998-05-12 | Skw Trostberg Aktiengesellschaft | Water-soluble polycondensation products based on amino-s-triazines and the use thereof |
| US6555683B1 (en) * | 1995-03-31 | 2003-04-29 | Skw Polymers Gmbh | Condensation products based on amino-s-triazines and the use thereof |
| US5798425A (en) * | 1995-04-07 | 1998-08-25 | Skw Trostberg Aktiengesellschaft | Co-polymers based on oxyalkyleneglycol alkenyl ethers and unsaturated dicarboxylic acid derivatives |
| US6139623A (en) * | 1997-01-21 | 2000-10-31 | W. R. Grace & Co.-Conn. | Emulsified comb polymer and defoaming agent composition and method of making same |
| US6506248B1 (en) * | 1997-04-10 | 2003-01-14 | James Hardie Research Pty Limited | Building products |
| US6187941B1 (en) * | 1997-09-06 | 2001-02-13 | Asta Medica Aktiengesellschaft | Process for the preparation of oxazaphosphorine-2-amines |
| US6777517B1 (en) * | 1999-06-11 | 2004-08-17 | Degussa Construction Chemicals Gmbh | Copolymers based on unsaturated mono-or dicarboxylic acid derivatives and oxyalkylene glycol alkenyl ethers, method for the production and use thereof |
| US7041167B2 (en) * | 2001-03-05 | 2006-05-09 | James Hardie International Finance B.V. | Low density accelerant and strength enhancing additive for cementitious products and methods of using same |
| US20050239924A1 (en) * | 2002-03-27 | 2005-10-27 | Lettkeman Dennis M | High molecular weight additives for calcined gypsum and cementitious compositions |
| US20050250888A1 (en) * | 2002-03-27 | 2005-11-10 | Lettkeman Dennis M | High strength flooring compositions |
| US20080108732A1 (en) * | 2004-10-15 | 2008-05-08 | Philipp Wieland | Polycondensation Product Base on Aromatic or Heteroaromatic Compounds, Method for the Production Thereof, and Use Thereof |
| US20060280970A1 (en) * | 2005-06-14 | 2006-12-14 | United States Gypsum Company | High strength flooring compositions |
| US20060281837A1 (en) * | 2005-06-14 | 2006-12-14 | United States Gypsum Company | Fast drying gypsum products |
| US20060278134A1 (en) * | 2005-06-14 | 2006-12-14 | Lettkeman Dennis M | Modifiers for gypsum slurries and method of using them |
| US20060278127A1 (en) * | 2005-06-14 | 2006-12-14 | United States Gypsum Company | Gypsum products utilizing a two-repeating unit dispersant and a method for making them |
| US20060280898A1 (en) * | 2005-06-14 | 2006-12-14 | United States Gypsum Company | Modifiers for gypsum slurries and method of using them |
| US20060278129A1 (en) * | 2005-06-14 | 2006-12-14 | Qingxia Liu | Effective use of dispersants in wallboard containing foam |
| US20060278130A1 (en) * | 2005-06-14 | 2006-12-14 | Qingxia Liu | Gypsum products utilizing a two-repeating unit dispersant and a method for making them |
| US20060281886A1 (en) * | 2005-06-14 | 2006-12-14 | Manfred Bichler | Polyether-containing copolymer |
| US20060280899A1 (en) * | 2005-06-14 | 2006-12-14 | United States Gypsum Company | Method of making a gypsum slurry with modifiers and dispersants |
| US20060278135A1 (en) * | 2005-06-14 | 2006-12-14 | Qingxia Liu | Method of making a gypsum slurry with modifiers and dispersants |
| US20080017078A1 (en) * | 2005-06-14 | 2008-01-24 | Manfred Bichler | Liquid admixture composition |
| US7070648B1 (en) * | 2005-06-16 | 2006-07-04 | Lyondell Chemical Technology, L.P. | Preparation of gypsum compositions |
| US20100197840A1 (en) * | 2007-07-31 | 2010-08-05 | Sika Technology Ag | Emulsifying polymers and their use |
| US20090101045A1 (en) * | 2007-10-23 | 2009-04-23 | Dennis Lettkeman | Gypsum mixtures for forming solids |
Cited By (27)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20140134407A1 (en) * | 2008-05-06 | 2014-05-15 | Intertape Polymer Corporation | Edge coating for tapes |
| US9328025B2 (en) * | 2010-12-30 | 2016-05-03 | United States Gypsum Company | Effective use of melamine sulfonate condensate dispersants in wallboard containing foam |
| US20120172468A1 (en) * | 2010-12-30 | 2012-07-05 | Blackburn David R | Effective use of melamine sulfonate condensate dispersants in wallboard containing foam |
| US9309152B2 (en) * | 2011-08-10 | 2016-04-12 | Sika Technology Ag | Process for drying concrete dispersants |
| US20140135427A1 (en) * | 2011-08-10 | 2014-05-15 | Sika Technology Ag | Process for drying concrete dispersants |
| WO2013117465A1 (en) * | 2012-02-08 | 2013-08-15 | Basf Se | Aqueous dispersion |
| CN104093678B (en) * | 2012-02-08 | 2016-09-07 | 巴斯夫欧洲公司 | Water-borne dispersions |
| AU2013218204A1 (en) * | 2012-02-08 | 2014-08-28 | Basf Se | Aqueous dispersion |
| CN104093678A (en) * | 2012-02-08 | 2014-10-08 | 巴斯夫欧洲公司 | Aqueous dispersion |
| US8883907B2 (en) * | 2012-02-08 | 2014-11-11 | Basf Se | Aqueous dispersion |
| AU2013218204B2 (en) * | 2012-02-08 | 2016-03-17 | Basf Se | Aqueous dispersion |
| US20130203926A1 (en) * | 2012-02-08 | 2013-08-08 | Bogdan Moraru | Aqueous dispersion |
| CN103011680B (en) * | 2012-12-13 | 2014-08-27 | 中国矿业大学(北京) | Sulfate-resistant organosilicone modified polycarboxylic superplasticizer and preparation method thereof |
| CN103011680A (en) * | 2012-12-13 | 2013-04-03 | 中国矿业大学(北京) | Sulfate-resistant organosilicone modified polycarboxylic superplasticizer and preparation method thereof |
| RU2689166C1 (en) * | 2013-12-20 | 2019-05-24 | Констракшн Рисёрч Энд Текнолоджи Гмбх | Additive for improvement of rheological properties of inorganic binding substances |
| CN103922637A (en) * | 2014-03-14 | 2014-07-16 | 石家庄市长安育才建材有限公司 | Water reducing agent for high performance C80 concrete, and preparation method and application method thereof |
| US10005694B2 (en) | 2014-10-31 | 2018-06-26 | Rohm And Haas Company | Two component synthetic water retention agent and rheology modifier for use in cements, mortars and plasters |
| WO2016069289A1 (en) | 2014-10-31 | 2016-05-06 | Rohm And Haas Company | Two component synthetic water retention agent and rheology modifier for use in cements, mortars and plasters |
| CN107383358A (en) * | 2017-07-17 | 2017-11-24 | 上海台界化工有限公司 | Hyperbranched synthetic method of unsaturated APEO and products thereof and application |
| CN111433171A (en) * | 2017-12-07 | 2020-07-17 | 建筑研究和技术有限公司 | Dispersant composition |
| CN108417836A (en) * | 2018-01-31 | 2018-08-17 | 闽南师范大学 | A kind of binders for electrodes of new type lithium ion battery and preparation method thereof |
| CN112313186A (en) * | 2018-06-21 | 2021-02-02 | 竹本油脂株式会社 | Additive for hydraulic composition |
| WO2020133777A1 (en) * | 2018-12-24 | 2020-07-02 | 科之杰新材料集团有限公司 | Phosphate type polycarboxylate water reducer and preparation method |
| CN111377645A (en) * | 2018-12-31 | 2020-07-07 | 江苏苏博特新材料股份有限公司 | Micromolecular phosphonic acid water reducing agent suitable for machine-made sand and preparation method thereof |
| CN111533844A (en) * | 2020-06-04 | 2020-08-14 | 江苏万邦新材料科技有限公司 | Modified concrete water-retaining agent and preparation method thereof |
| CN113882155A (en) * | 2020-07-01 | 2022-01-04 | 中国石油化工股份有限公司 | Polyethylene glycol maleimide-styrene copolymer sizing agent and preparation method and application thereof |
| CN111925488A (en) * | 2020-08-11 | 2020-11-13 | 陈兴涛 | Shrinkage-reducing polycarboxylate superplasticizer and preparation method thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| TW201116501A (en) | 2011-05-16 |
| CA2773145A1 (en) | 2011-03-10 |
| US20140073711A1 (en) | 2014-03-13 |
| US20130005861A1 (en) | 2013-01-03 |
| US8597426B2 (en) | 2013-12-03 |
| US20110054053A1 (en) | 2011-03-03 |
| AR078255A1 (en) | 2011-10-26 |
| MX2012002325A (en) | 2012-05-23 |
| WO2011028817A1 (en) | 2011-03-10 |
| CA2773145C (en) | 2017-01-03 |
| US8801852B2 (en) | 2014-08-12 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP2473457B1 (en) | Formulation and its use | |
| US20110054081A1 (en) | Formulation and its use | |
| EP2181079B1 (en) | A liquid admixture composition | |
| JP6025731B2 (en) | Dispersant containing gypsum slurry | |
| JP4531044B2 (en) | Cement admixture, cement composition and method for its construction, and method for producing hardened cement | |
| RU2567258C2 (en) | Dispersant | |
| JP2014503455A (en) | Additives for building material mixtures containing superplasticizers | |
| EP1434745A1 (en) | Superplasticizer for concrete and self-leveling compounds | |
| JP2008133176A (en) | Cement admixture | |
| US8344084B2 (en) | Liquid admixture composition | |
| US8349979B2 (en) | Liquid admixture composition | |
| JP2008105867A (en) | Cement admixture | |
| JP2016179926A (en) | Cement admixture and cement composition | |
| JP4448274B2 (en) | Method of constructing cement admixture and cement composition | |
| JP2019112249A (en) | Admixture for concrete highly containing scm additive agent, admixture-containing composition and cement composition containing the same | |
| EP4330207A1 (en) | Cement dispersant comprising a naphthalenesulfonic acid polycondensate and at least one of a phosphorylated polycondensate and a polycarboxylate ether, and construction composition | |
| JP2008105868A (en) | Cement admixture | |
| AU2002336519A1 (en) | Superplasticizer for concrete and self-leveling compounds |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: BASF CONSTRUCTION POLYMERS GMBH, GERMANY Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:DIERSCHKE, FRANK;KRAUS, ALEXANDER;SIGNING DATES FROM 20090901 TO 20090902;REEL/FRAME:023188/0418 |
|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |